(UroToday.com) The 2024 European Association of Urology (EAU) annual congress held in Paris, France was host to a plenary session addressing imaging-related controversies for the staging of genitourinary cancers. Dr. Brian Chapin discussed the potential role of biomarkers in improving disease characterization.
Dr. Chapin began by noting that the current risk stratification paradigm for prostate cancer relies heavily on stage, histology, and biology.
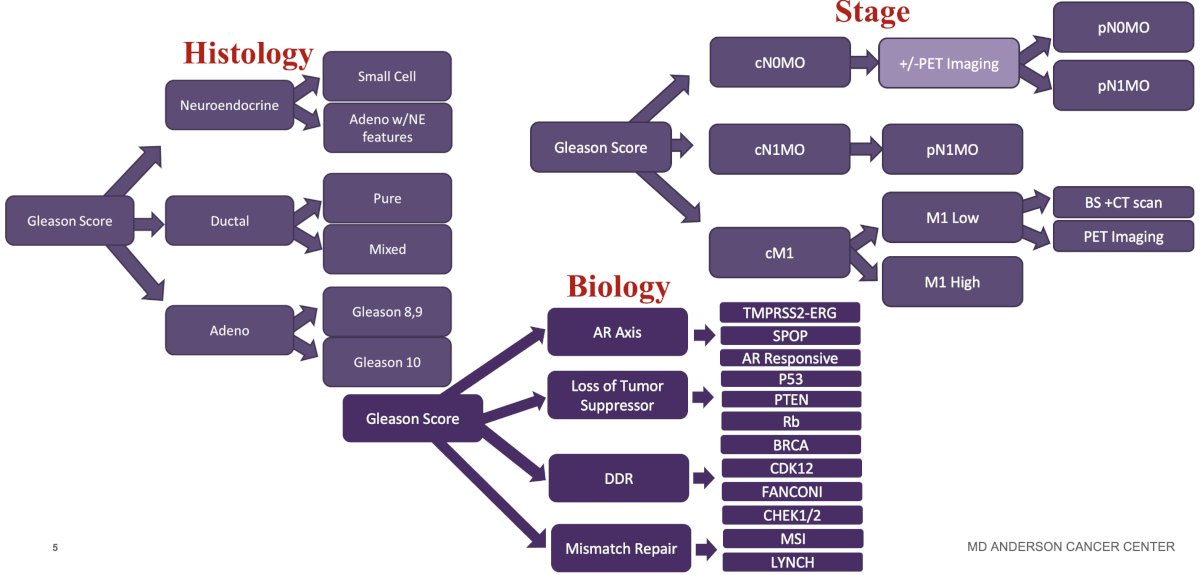
This risk stratification schema relies on compartmentalization, which can be leveraged to:
- Balance arms in clinical trials
- Stratification factors (M1a/b vs M1c, high vs low volume, prior local therapy, etc.)
- Balance groups in retrospective studies (matching, propensity scoring)
- Single center: level the field
- NCDB, SEER: Unknown confounders making balancing a challenge
But can biomarkers provide added value to the current risk stratification landscape? The currently available biomarkers can be used across various settings:
- Pre-biopsy
- Post-biopsy
- Positive biopsy
- Post-radical prostatectomy
- Metastatic

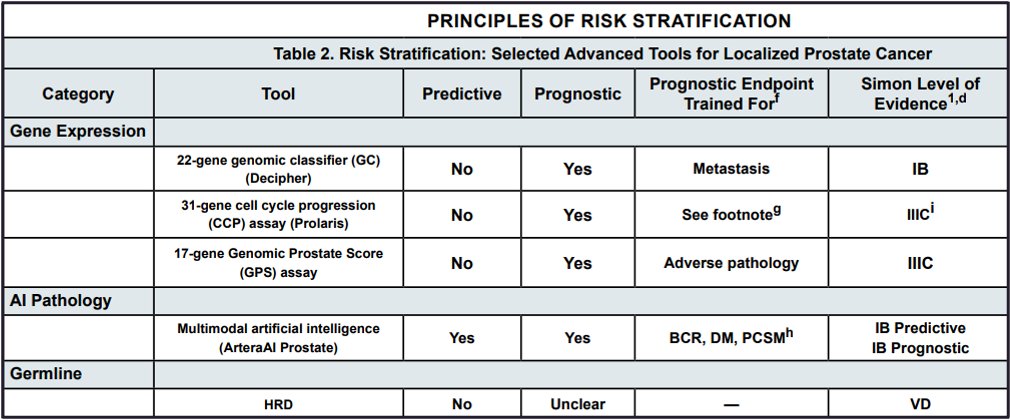
To date, contemporary guidelines do not routinely recommend the use of biopsy biomarkers (e.g. Decipher, Onctotype, Prolaris, ArteraAI). The EAU recommends that such biomarkers not be offered routinely. However, they can be considered in subsets of patients, where such tests may provide clinically actionable information (e.g., consideration of active surveillance for favorable intermediate risk patients). Conversely, the recently updated 2024 NCCN guidelines provide a more ‘nuanced’ approach and level of evidence for individual biomarkers in this setting. ArteraAI Prostate is the first predictive biomarker in this setting (addition of short-term ADT to radiotherapy for intermediate-risk patients).1 Additionally, Decipher® is now assigned a 1B level of evidence for use in clinical practice for prognosticating the underlying risk of metastasis.
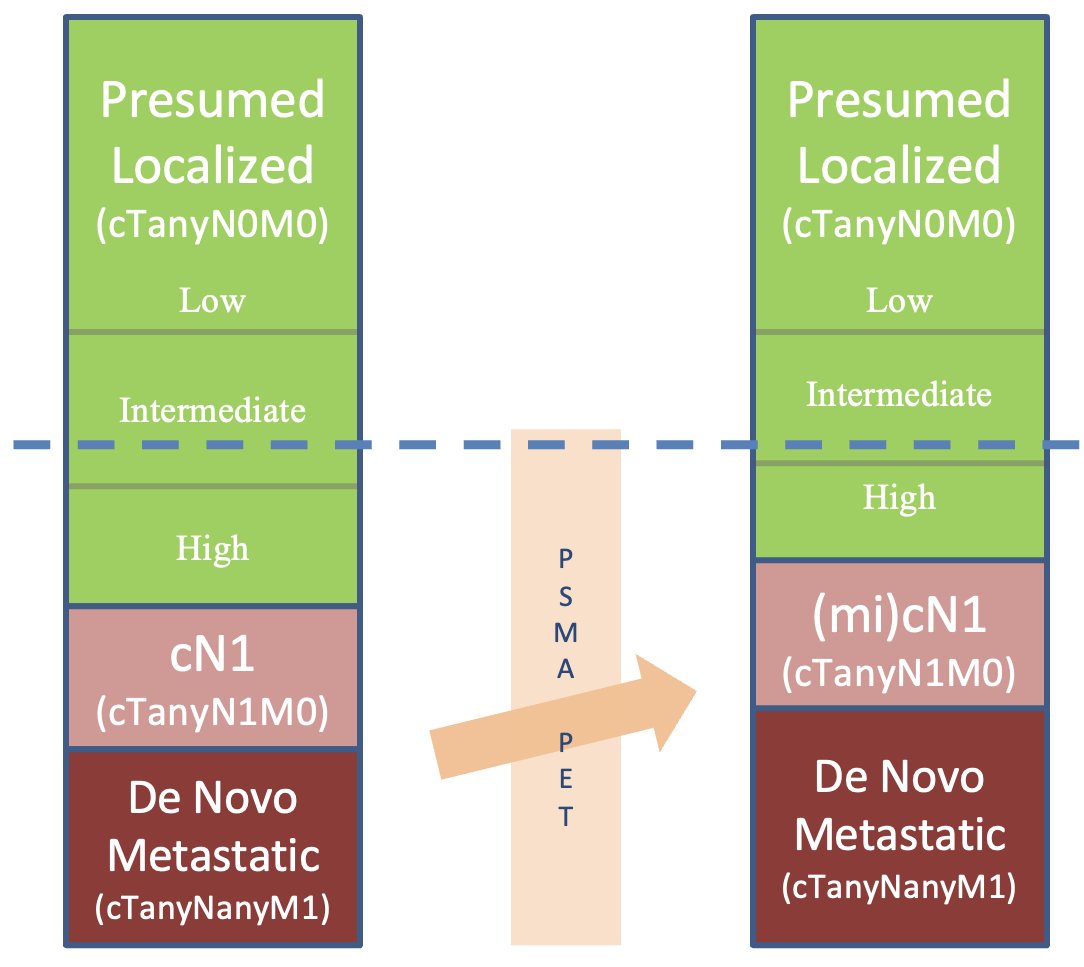
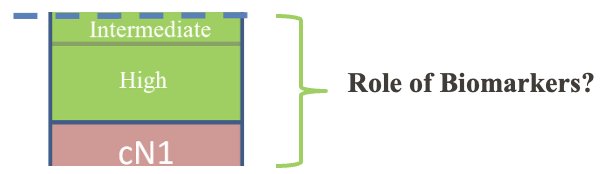
Where will biomarkers end up ‘fitting in’ in the current de novo castrate-sensitive disease landscape? The staging paradigm has evolved considerably with the increased utilization of PSMA-PET/CT. The proportion of patients with presumed localized disease continues to shrink with the improved sensitivity of PSMA-PET/CT for the detection of nodal and metastatic disease. As such, he argued that the subset of patients for whom genetic testing is likely to result in clinically actionable differences is likely those patients with intermediate/high-risk and cN1 disease.
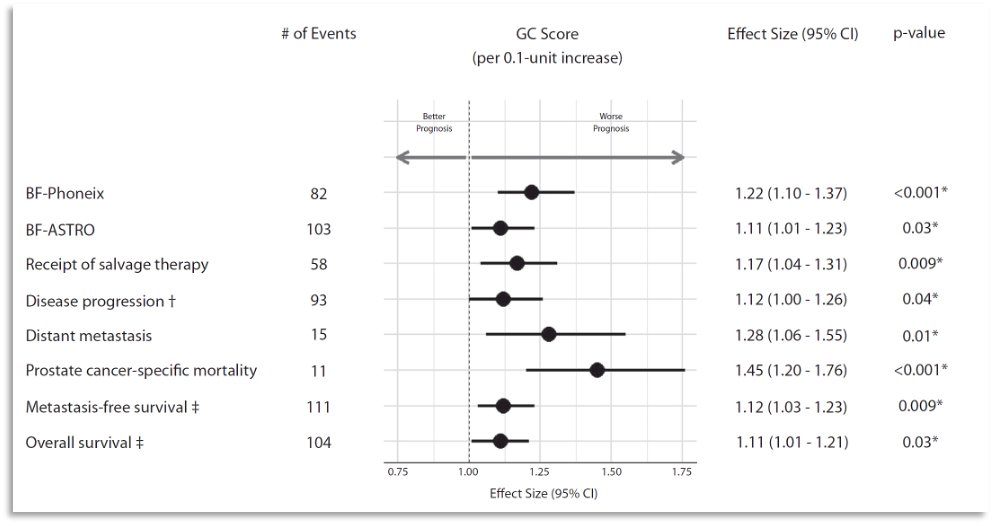
What is the current evidence for genomic biomarkers in these patients? In 2023, Spratt et al published the results of an analysis evaluating the performance of Decipher® in men with intermediate-risk disease enrolled in NRG Oncology/RTOG 01-26, which was a randomized phase 3 trial of men with intermediate-risk prostate cancer randomized to 70.2 Gy versus 79.2 Gy of radiation therapy without androgen deprivation therapy. At a median follow-up of 12.8 years, the 22-gene genomic classifier (per 0.1 unit) was independently prognostic for:
- Disease progression (sHR: 1.12; p=0.04)
- Biochemical failure (sHR: 1.22; p<0.001)
- Distant metastasis (sHR: 1.28; p=0.01)
- Prostate cancer-specific mortality (sHR: 1.45; p<0.001)
Ten-year distant metastasis in genomic classifier low-risk patients was 4% compared with 16% for high-risk patients.2
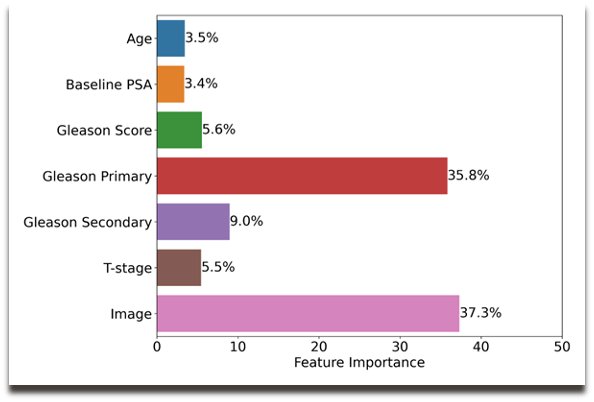
Results of the ArteraAI validation analysis were similarly published in 2023. The ArteraAI model was developed using digital pathology images from pre-treatment prostate tissue and clinical data from 5,727 patients enrolled in four phase 3 randomized trials, in which treatment was radiotherapy with or without ADT (NRG/RTOG 9202, 9413, 9910, 0126), and subsequently validated in a firth trial (NRG/RTOG 9408) after the model was locked. NRG/RTOG 9408 evaluated radiotherapy +/- 4 months of ADT in intermediate-risk patients. The primary endpoint for this model was distant metastases. The model used baseline data to provide a binary output that a given patient will likely benefit from ADT or not. The ArteraAI model relies on the multi-modal variables:
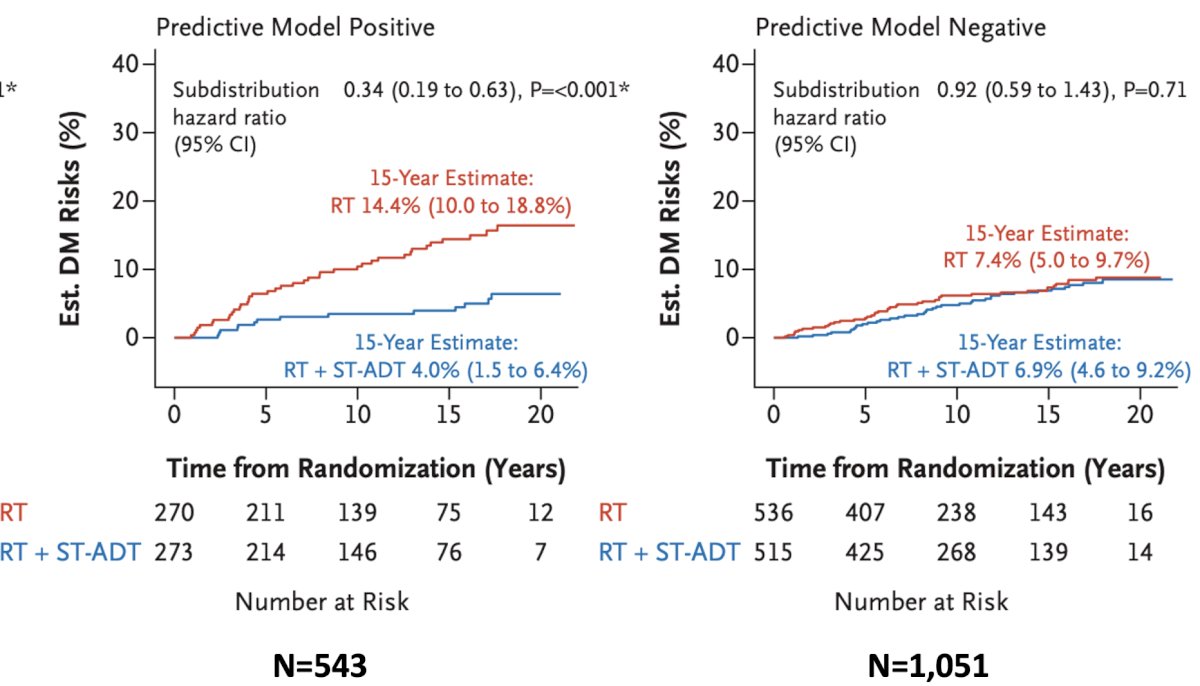
Significantly, this model was able to predict responders to short-term ADT, whereby patients who were model positive derived a benefit from short-term ADT (15-year distant metastasis rate: 14.4% versus 4%), whereas those who were predictive model negative did not derive a benefit (7.4% versus 6.9%).1
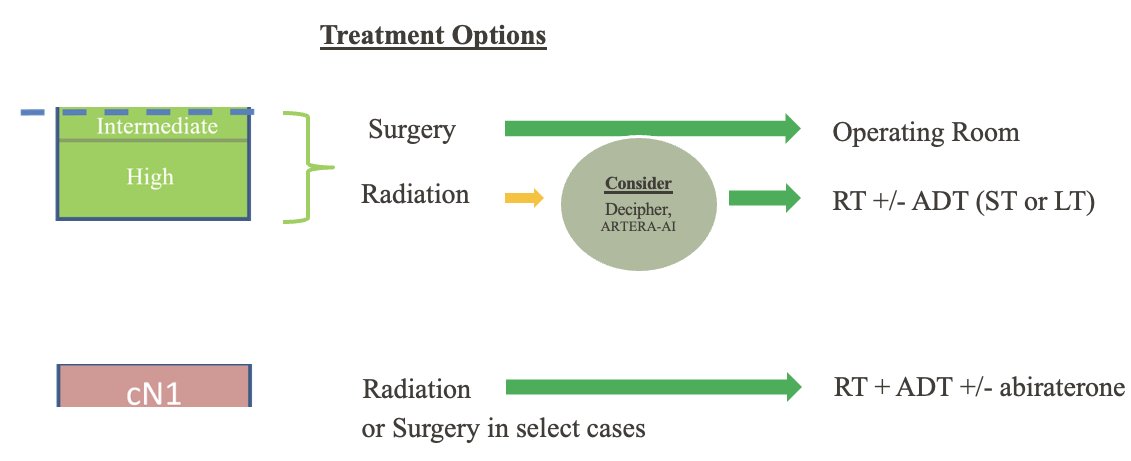
Based on the current evidene, Dr. Chapin argued that the use of these biomarkers can only be recommended for patients with intermediate and high-risk disease, considered for radiation +/- short- or long-term ADT.
Biomarkers are increasingly incorporated into ongoing clinical trials. The NRG-GU009 PREDICT-RT clinical trial is incorporating Decipher testing for treatment de-intensification in NCCN high-risk patients considered for radiotherapy.

The Genomic Umbrella Neoadjuvant Study (GUNS; NCT04812366) trial is utilizing a biomarker-targeted approach to neoadjuvant treatment intensification for high-risk prostate cancer patients.
Dr. Chapin concluded his presentation as follows:
- It is important to differentiate between prognostic and predictive biomarkers
- Prospective trials and/or trials with a generation of biobanks will be important to:
- Allow for validation of current biomarkers
- Test new biomarkers for prognostic risk stratification
- Prospectively assess predictive biomarkers with defined therapeutic benefit
- Skeptical optimism is appropriate until validations complete
Presented by: Brian Chapin, MD, Associate Professor, Department of Urology, The University of Texas MD Anderson Cancer Center, Houston, TX
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th – April 8th, 2024References:
- Spratt DE, Tang S, Sun Y, et al. Artificial Intelligence Predictive Model for Hormone Therapy Use in Prostate Cancer. NEJM Evid 2023;2(8).
- Spratt DE, Liu VY, Michalski J, et al. Genomic Classifier Performance in Intermediate-Risk Prostate Cancer: Results From NRG Oncology/RTOG 0126 Randomized Phase 3 Trial. Int J Radiat Oncol Biol Phys. 2023;117(2): 370-377.


