(UroToday.com) The 2024 European Association of Urology (EAU) annual meeting featured a joint session of the EAU and the CAU and a presentation by Dr. Wolfgang Fendler discussing an update on PSMA PET/CT, specifically when its use in staging has an impact on survival.
Dr. Fendler started by highlighting that PSMA PET has led to a considerable stage shift for prostate cancer. This includes (i) higher sensitivity and equal specificity versus CT + bone scan in the initial setting, (ii) 76% detection, 84% PPV, and 46% management impact in the mHSPC setting, (iii) 55% M1 up-staging for nmCRPC, and (iv) up- and down-staging for eligibility of PSMA radioligand therapy in mCRPC: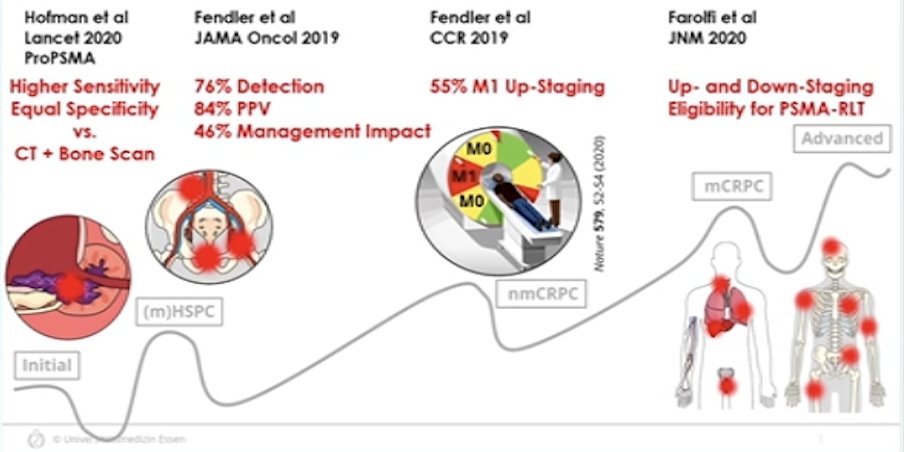
With regards to the current evidence for the impact of PSMA PET on overall survival, there is a lack of RCTs. As of 2023, EAU – EANM – ESTRO – ESUR stated “Treatment should not be changed based on PSMA PET/CT findings, in view of currently available data.” However, in 2024, this was changed to “Results from RCTs (…) are awaited before a recommendation can be made to treat patients based on the results of these tests.”
The PRIMORDIUM trial provides treatment guidance and randomizing men with initial high-risk prostate cancer (biochemical recurrence after radical prostatectomy and PSA doubling time <= 12 months) to salvage radiotherapy + an LHRH agonist +/- apalutamide in the setting of a positive PSMA PET. For those with a negative PSMA PET, they are being observed. The primary endpoint is PSMA PET metastatic PFS with several secondary endpoints, including time to PSA progression, PSA response rate, PSA and testosterone levels at week 26, time to loco-regional progression by PSMA-PET, and overall survival:
Dr. Fendler also notes that there is upcoming evidence for PSMA PET and survival with regards to PSMA radioligand therapy, metastasis-directed therapy, salvage radiotherapy, and curative intent radiotherapy.
In a sub-study of the VISION trial, Kuo and colleagues previously presented at ASCO 2022 and EANM 2023 that PSMA overexpression by tumor SUVmean is associated with rPFS and overall survival. In more detail, higher whole-body SUVmean was associated with improved clinical outcomes: those patients in the highest quartile (SUVmean: rPFS, ≥ 10.2; OS, ≥ 9.9) had a median rPFS and OS of 14.1 and 21.4 months, vs 5.8 and 14.5 months for those in the lowest quartile (< 6.0; < 5.7), respectively.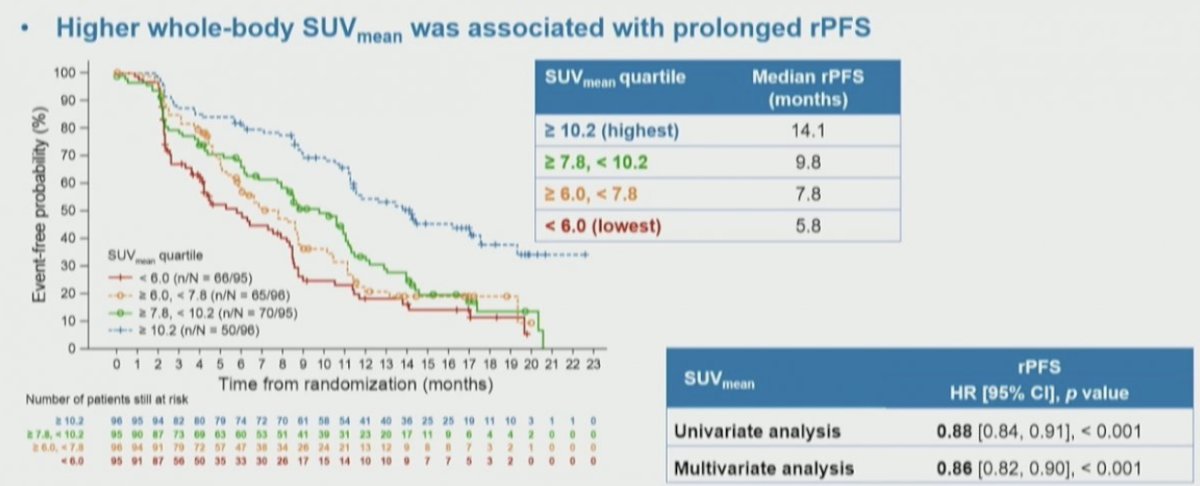
The authors then assessed the association of these quartiles of whole body SUVmean with overall survival, again finding a significant stratification between the groups. Notably, among these patients who received 177Lu-PSMA-617, those with the highest SUVmean levels had the longest overall survival: 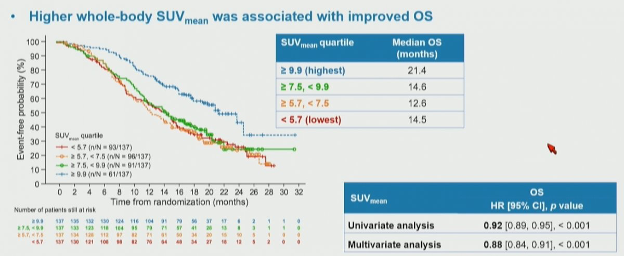
With regards to metastasis-directed therapy, Dr. Fender notes that the ORIOLE trial1 randomized 54 men in a 2:1 ratio to receive stereotactic body radiotherapy or observation. The primary endpoint for this trial was progression at 6 months, defined as a PSA increase, radiographic or symptomatic progression, ADT initiation, or death. Progression at 6 months occurred in 7 of 36 patients (19%) receiving stereotactic body radiotherapy and 11 of 18 patients (61%) undergoing observation (p = 0.005). Furthermore, treatment with stereotactic body radiotherapy improved median progression-free survival (not reached vs 5.8 months; HR 0.30, 95% CI 0.11-0.81; p = 0.002):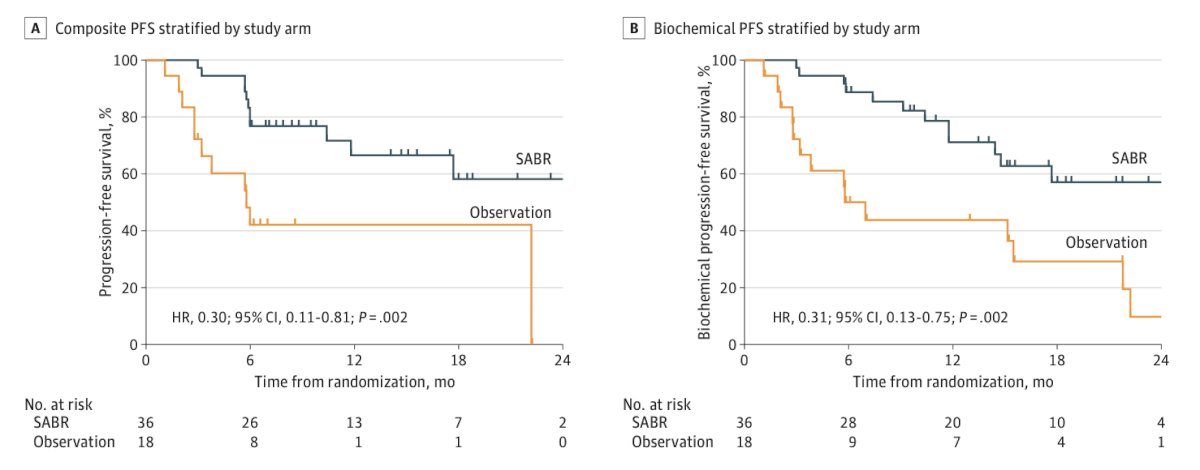
Additionally, the proportion of men with no untreated PMSA PET lesions with progression at 6 months was 1 of 19 (5%; 95% CI, 0-26.8) compared with 6 of 16 (38%; 95% CI, 18.5-61.5) for those with any untreated lesions (p = 0.03). The median PFS was unreached among participants with no untreated lesions vs 11.8 months among participants with any untreated lesions (HR 0.26, 95% CI 0.09-0.76; p = 0.006):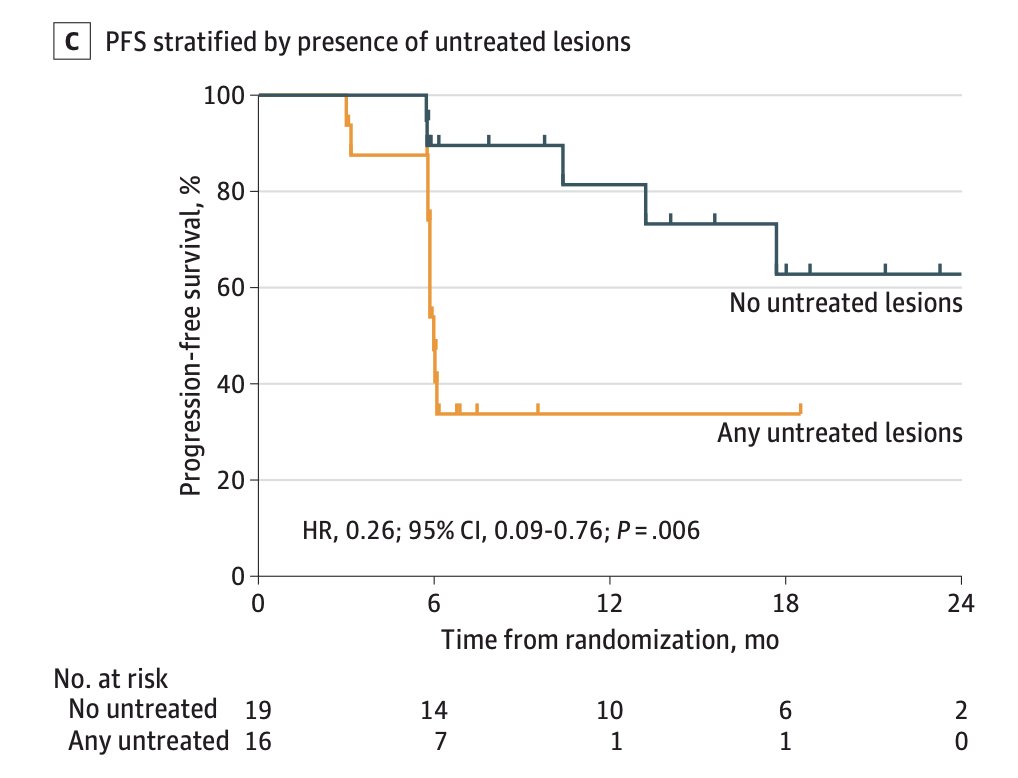
Dr. Fendler then discussed the EMPIRE-1 trial,2 which is a single-center, open-label, phase 2/3 randomized controlled trial of patients with prostate cancer with detectable PSA after prostatectomy and negative conventional imaging (no extrapelvic or bone findings). Patients were randomly assigned in a 1:1 ratio to radiotherapy directed by conventional imaging alone or to conventional imaging plus 18F-fluciclovine-PET/CT. The primary endpoint was 3 year event-free survival, with events defined as biochemical or clinical recurrence or progression, or initiation of systemic therapy. There were 165 patients randomly assigned, with a median follow-up of 3.52 years (95% CI 2.98-3.95). The 3 year event-free survival rate was 63.0% (95% CI 49.2-74.0) in the conventional imaging group versus 75.5% (95% CI 62.5-84.6) for 18F-fluciclovine-PET/CT (difference 12.5; 95% CI 4.3-20.8; p=0.0028):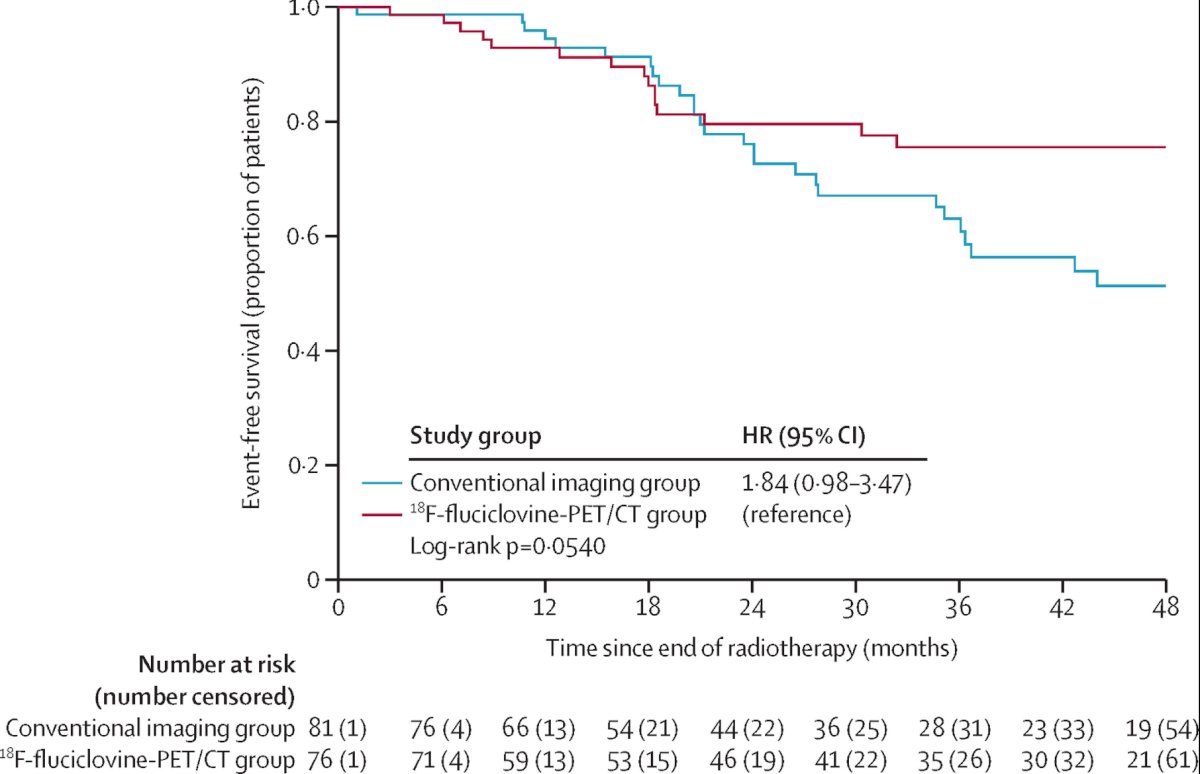
The final trial discussed was the PSMA-SRT trial, a randomized prospective phase III trial of 68Ga-PMSA-11 PET/CT salvage radiotherapy planning.3 In this trial, there were 193 patients enrolled from September 6, 2018, to August 17, 2020: 90 were randomized to the control group, and 103 were randomized to the PSMA group: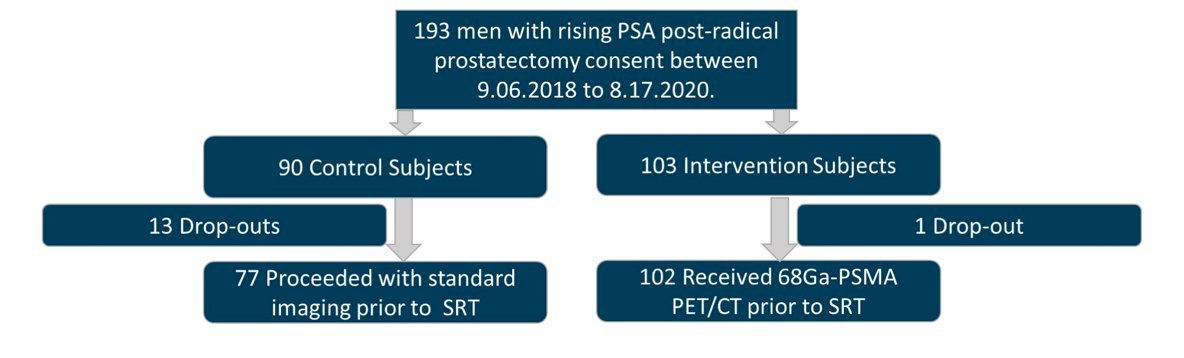
The median time from radical prostatectomy to enrollment and median PSA was 20.3 months (IQR 1.4–245) and 0.3 ng/ml (IQR 0.2-10.3) for the control group, and 28.3 months (IQR 1.2–21) and 0.23 ng/ml (IQR 0.1-29.9) for the PSMA group. PSMA was positive in 38/102 (38%): 12/102 (12%) outside of the pelvis, and 20/102 (20%) in pelvic lymph nodes. Pre-randomization radiotherapy plan and delivered radiotherapy plan were available in 77/90 control (86%) and 102/103 PSMA (99%) patients (p = 0.0004), respectively. There were 0/77 (0%) and 7/102 (7%) minor changes in the control and PSMA groups (p = 0.02). There were 17/77 (22%) and 46/102 (45%), major changes (p = 0.004), with 33/45 (73%) being PSMA-related. The primary endpoint of the trial is the success rate of salvage radiotherapy measured as biochemical progression-free survival, with patients being followed for 5 years.
Finally, Dr. Fendler noted the multicenter PROMISE registry that is a collaboration between the EAU and Young Academic Urologists (YAU), with an aim to establish the prognostic value of PSMA PET for various stages of prostate cancer for more accurate risk assessment. To date, there are 1,611 enrolled patients, with a median overall survival follow-up of 7 years.
Dr. Fendler concluded his presentation discussing an update on PSMA PET/CT, specifically when its use in staging has an impact on survival, with the following conclusions:
- PSMA PET has become standard imaging in nearly all stages of the disease
- There has been an impact on management, particularly for biochemical recurrence and CRPC
- There is no level 3 evidence for survival impact as of yet
- Future settings for PSMA guidance:
- PSMA radioligand therapy
- Metastasis directed therapy
- Salvage radiotherapy
- RCT data is urgently needed
Presented by: Wolfgang Fendler, MD, Vice Chair of Nuclear Medicine, University of Essen, Essen, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, WellStar MCG Health, @zklaassen_md on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
References:
- Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol 2020 Mar 26;6(5):650-659.
- Jani AB, Schreibmann E, Goyal S, et al. 18F-fluciclovine-PET/CT imaging versus conventional imaging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE-1): A single centre, open-label, phase 2/3 randomized controlled trial. Lancet. 2021 May 22;397(10288):1895-1904.
- Calais J, Czernin J, Fendler WP, et al. Randomized prospective phase III trial of 68Ga-PSMA-11 PET/CT molecular imaging for prostate cancer salvage radiotherapy planning [PSMA-SRT]. BMC Cancer 2019 Jan 7;19(1):18.


