(UroToday.com) The 2024 European Association of Urology (EAU) annual meeting featured a plenary session highlighting risk adapted screening for prostate cancer in Europe and a presentation by Dr. Caroline Moore discussing the UK ReImagine trial. Dr. Moore notes that the reason to screen for prostate cancer is that it reduces prostate cancer death, including a 20% reduction in mortality at 16 years, however at the risk of too many low risk cancer diagnoses, and too many unnecessary biopsies.
In the UK, men with a PSA > 3 ng/mL receive an MRI. The PRECISION trial showed that MRI-targeted vs standard biopsy reduces the overdiagnosis of low risk prostate (5% of newly diagnosed men have Gleason 3+3),1 however the age-adjusted prostate cancer mortality (per 100,000 person years) in the UK is still too high compared to other countries:
- UK: 12.4
- USA: 8.2
- France: 8.4
- Spain: 7.3
- Italy: 5.9
Additionally, black men have twice the risk of prostate cancer than white men, with a 1 in 4 risk of prostate cancer, and a 1 in 12 risk of prostate cancer death. Dr. Moore emphasized that what we want from modern screening is to:
- Maximize benefit
- Reduce prostate cancer deaths by finding the most harmful cancers
- Use the best tests available
- Minimize harm
- Reduce overdiagnosis, which reduces anxiety and unnecessary re-testing
- Reduces over treatment
Dr. Moore highlighted the Prostagram study, which is a blinded, paired screen positive trial of two screening centers. Participants include 411 men aged 50-69 years invited for screening from 7 primary care practices and community recruitment. The following highlights the diverse ethnicity in the Prostagram study: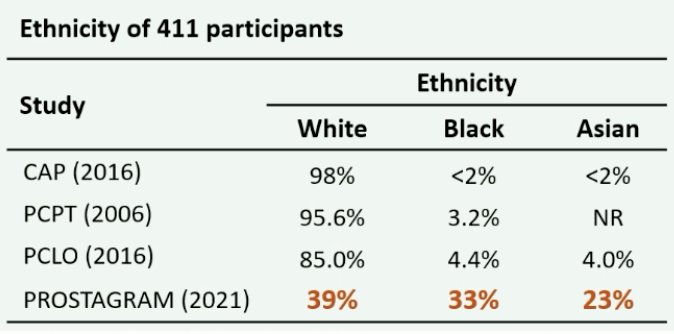
As follows is the full study design for Prostagram: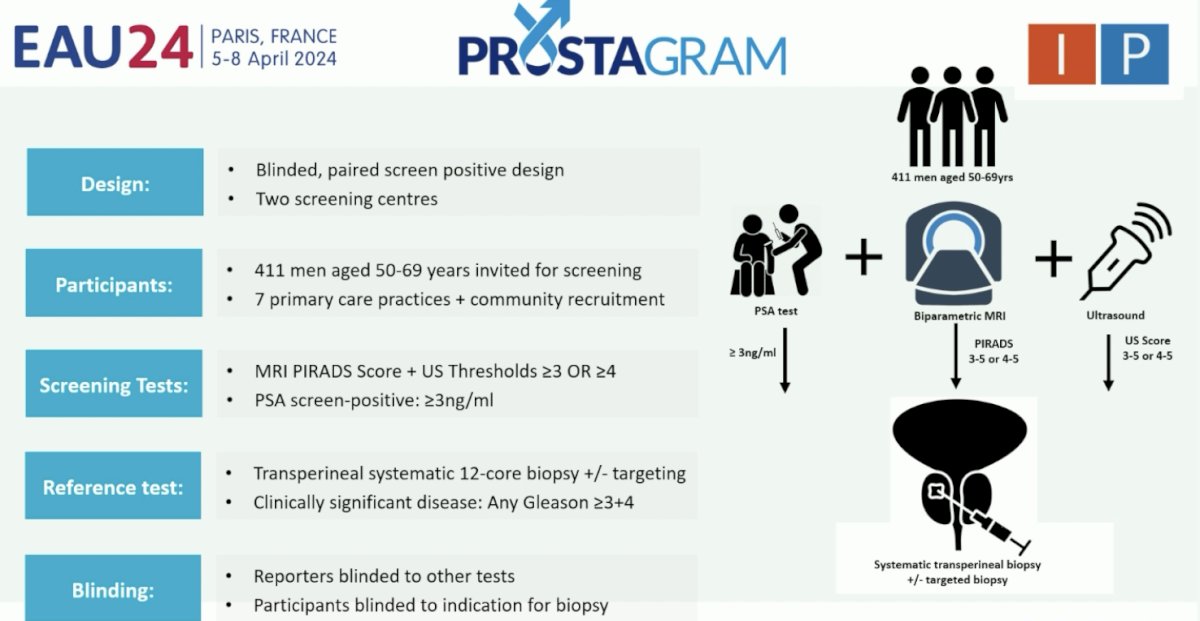
For Prostagram, PSA screening among those with PSA >= 3.0 ng/mL, the biopsy rate was 10%, sensitivity 41%, and specificity 81%, whereas in the bp-MRI group the following highlights the key findings:
- PIRADS 3: biopsy rate 18%, sensitivity rate 88%, specificity 51%
- PIRADS 4: biopsy rate 10.5%, sensitivity 65%, specificity 82%
The second trial Dr. Moore discussed was the UK ReImagine trial,2 whereby a screening PSA density is positive if > 0.12 ng/mL/mL or a bp-MRI is positive. Subsequently, each of these groups receive an NHS multiparametric MRI before a decision for biopsy is made. The following table highlights who responded to the invitation in ReImagine, including men aged 65-70 most likely to respond, black men having 20% the response rate of white men, and no difference across the index of multiple deprivations: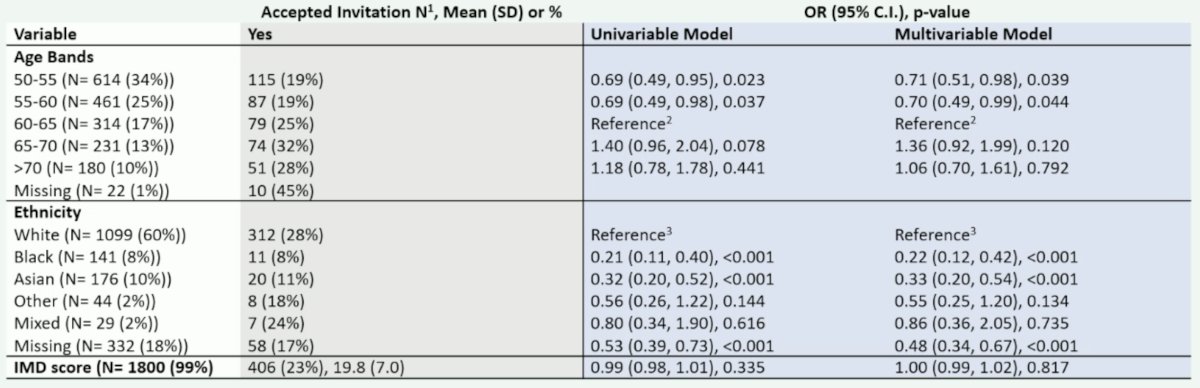
Among men with a PSA density > 0.12 ng/mL/mL, 1 in 20 men (16/303) had a raised PSA density alone, and 1 in 4 men had clinically significant prostate cancer. In men with bp-MRI, 1 in 6 men (48/303) had a positive screen, with 1 in 2 having clinically significant prostate cancer. The PSA distribution by screening MRI result is as follows, with over half of men with a clinically significant prostate cancer found on MRI having a PSA < 3 ng/mL: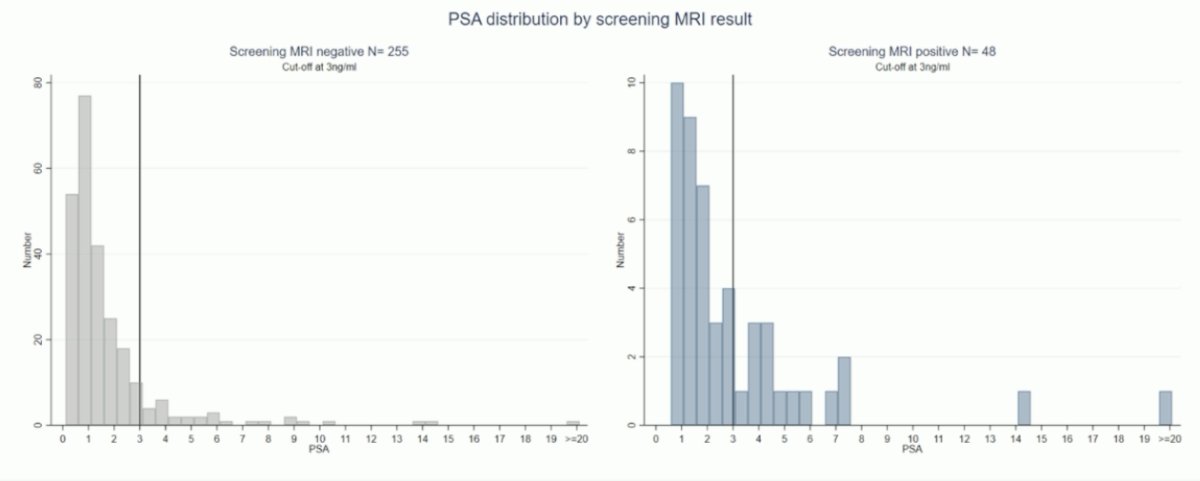
Of the 25 MRI detected cancers, two were Gleason 3+3, 19 were Gleason 3+4, two were Gleason 4+3, and three were Gleason 4+5. Among the five PSA density alone detected cancer, one was Gleason 3+3, 3 were Gleason 3+4, and one was Gleason 4+3.
Dr. Moore concluded her presentation discussing the UK ReImagine trial with the following conclusions:
- Black men had 1/5th the response rate of white men
- 1 in 6 men had a positive screening MRI, and 1 in 2 of these had clinically significant prostate cancer
- 1 in 20 men with a negative MRI had a raised PSA density, and 1 in 4 of these had clinically significant prostate cancer
- Over half of those with clinically significant prostate cancer had a PSA < 3 ng/mL
Presented by: Professor Caroline Moore, University College London, London, UK
Written by: Zachary Klaassen, MD, MSc - Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, WellStar MCG Health, @zklaassen_md on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
References:
- Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate cancer diagnosis. N Engl J Med 2018;378(19):1767-1777.
- Moore CM, Frangou E, McCartan N, et al. Prevalence of MRI lesions in men responding to a GP-led invitation for a prostate health check: A prospective cohort study. BMJ Oncology Aug 2023;2(1). e000057.


