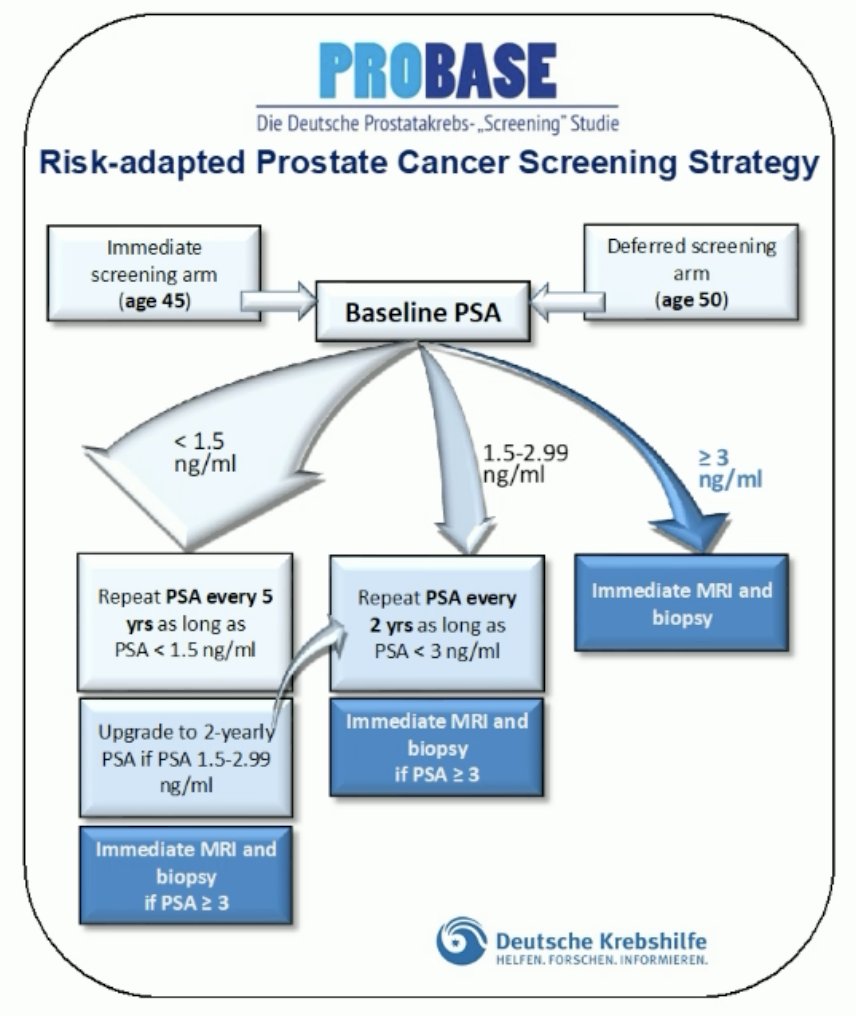
(UroToday.com) The 2024 European Association of Urology (EAU) annual meeting featured a plenary session highlighting risk adapted screening for prostate cancer in Europe and a presentation by Dr. Boris Hadaschik discussing the German PROBASE screening trial of young men. This trial started in 2014 and will be finished in 2034, enrolling more than 46,000 men. All men receive a baseline PSA at 45 or 50 years of age, with the following downstream surveillance algorithm:
- PSA < 1.5 ng/mL PSA in 5 years
- PSA 1.5-2.99 ng/mL PSA in 2 years
- PSA >= 3 ng/mL repeat PSA in 2 weeks, if PSA >= 3 ng/mL MRI + biopsy
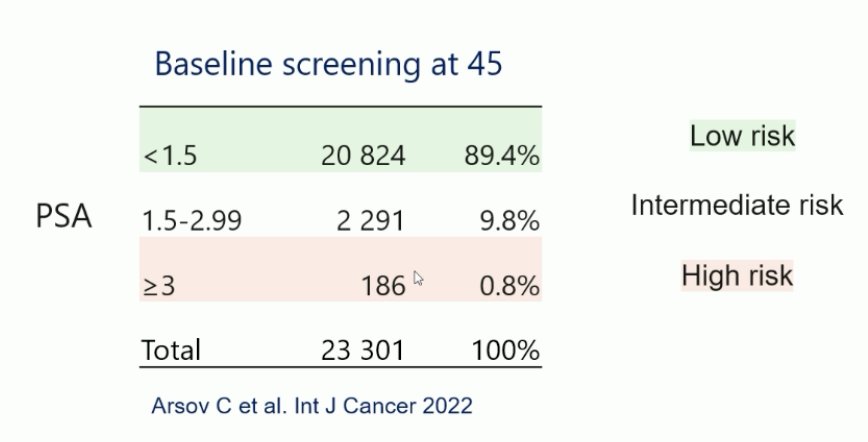
The baseline PSA screening results for patients 45 years of age are as follows:
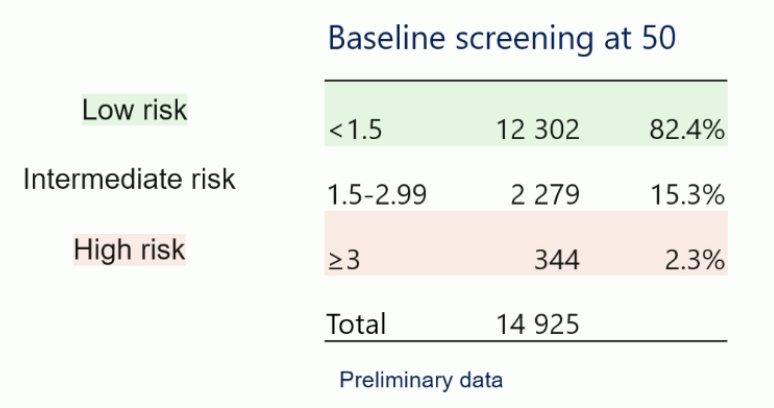
Among the men 45 years of age, a total of 48 prostate cancer cases were detected (overall prevalence 0.2%), of which 15 were ISUP Grade Group 1, 29 were ISUP Grade Group 2, and only four were ISUP Grade Group >= 3. Thus, the prevalence of screen-detected aggressive prostate cancer in 45-year-old men is very low. The baseline PSA screening results for patients 50 years of age are as follows:
Several publications have recently been published regarding the PROBASE trial, which Dr. Hadaschik overviewed as part of his concluding comments. The first results were published in 2023,1 finding that among a PSA invitation at 5 years, 79% were low risk and 70% deferred the screening arm. Among PSA tests outside the protocol, 11% were low risk and 25% deferred the screening arm. Overall, biopsy acceptance was 64%, including 71% with PSA >= 4 ng/mL, and 72% with PI-RADS 3-5. The second publication showed that screening 45 year old men with a digital rectal examination (DRE) is not useful, with a DRE acceptance of only 37%.2 Among 6,537 men that underwent DRE at age 45, a suspicious DRE was found in only 1% (57 out of 6,537), with only 3 prostate cancers found (cancer detection rate = 0.05%, two ISUP Grade Group 1, and one ISUP Grade Group 2). Finally, the third publication showed that prostate MRI in a young screening population is difficult to read,3 with moderate interobserver reliability between local and reference PI-RADS scores (k = 0.41). Additionally, a biopsy alone in PI-RADS >=4 (reference reading) had a NPV of 91%, leading to a reduction in negative biopsies by 68%, and avoiding detection of nonsignificant prostate cancer in 71% of cases.
Presented by: Boris A. Hadaschik, MD, Director and Chair, Department of Urology, University of Essen, Essen, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th – April 8th, 2024
References:
- Krilaviciute A, Albers P, Lakes J, et al. Adherence to a risk-adapted screening strategy for prostate cancer: First results of the PROBASE trial. Int J Cancer. 2023 Mar 1;152(5):854-864.
- Krilaviciute A, Becker N, Lakes J, et al. Digital Rectal Examination is not a Useful Screening Test for Prostate Cancer. Eur Urol Oncol. 2023 Dec;6(6):566-573.
- Boschheidgen M, Albers P, Schlemmer HP, et al. Multiparametric Magnetic Resonance Imaging in Prostate Cancer Screening at the Age of 45 Years: Results from the First Screening Round of the PROBASE Trial. Eur Urol. 2024 Feb;85(2):105-111.