(UroToday.com) The 2024 European Association of Urology (EAU) annual meeting featured a session on metastatic prostate cancer, and a presentation by Dr. Thomas Zilli discussing 24-month toxicity results of the randomized phase II PEACE V trial of salvage treatment of oligoRecurrent nodal prostate cancer Metastases (STORM). No standard treatment exists for prostate cancer pelvic nodal recurrences, metastasis directed therapy, or elective nodal pelvic radiotherapy being both valid treatment options. In a previous report, Dr. Zilli and colleagues demonstrated that no clinically meaningful differences were observed in worst grade 2 acute gastrointestinal or genitourinary toxicity or in quality of life subdomains between metastasis directed therapy and elective nodal pelvic radiotherapy during the first 3 months of follow-up. At the EAU 2024 annual meeting, Dr. Zilli and colleagues reported the first 24-month late toxicity results of the PEACE V-STORM randomized phase II clinical trial comparing these two treatment modalities.
STORM is an international, phase II, open-label, randomized, superiority trial (NCT03569241). Patients diagnosed with PET-detected pelvic nodal oligorecurrence (≤5 nodes) following radical local treatment for prostate cancer, were randomized in a 1:1 ratio between arm A: metastasis directed therapy and 6 months of ADT, or arm B: elective nodal pelvic radiotherapy (25 x 1.8 Gy) with metastasis directed therapy and 6 months of ADT. In the case of radiotherapy, SBRT (3 x 10 Gy) was used for arm A, with a simultaneous integrated boost in arm B. The trial design for the STORM trial is as follows:
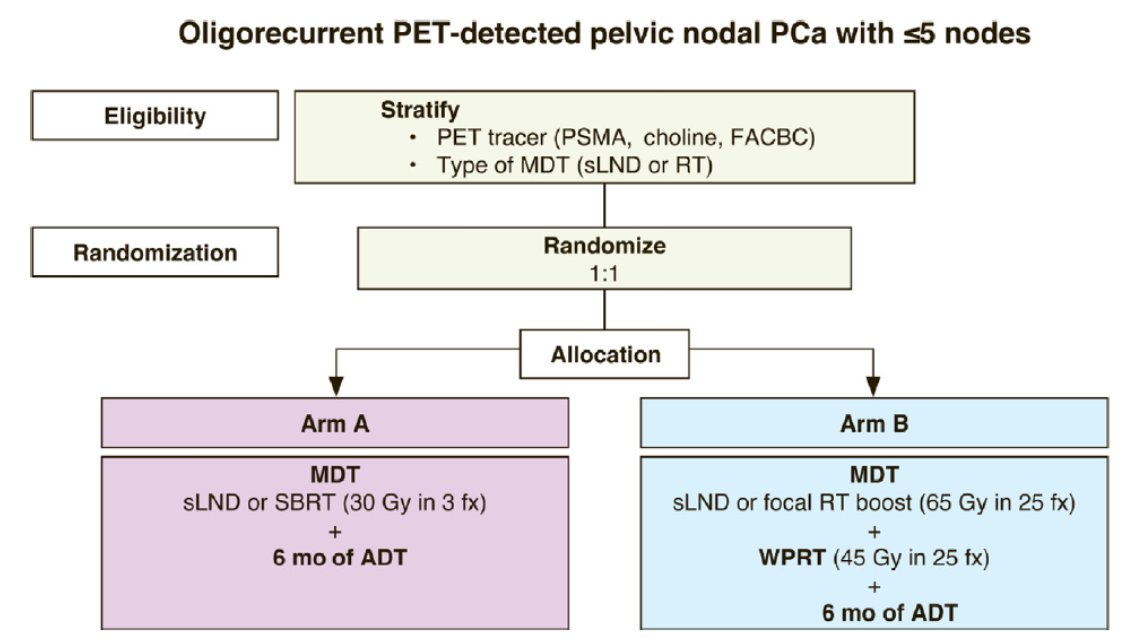
The primary endpoint is metastasis-free survival and the secondary endpoint is late toxicity, defined as worst grade ≥ 2 CTCAE v4.0 gastrointestinal and genitourinary toxicity exceeding baseline within 24 months of treatment. Quality of life was assessed using the EORTC QLQ C30 and PR25 questionnaires.
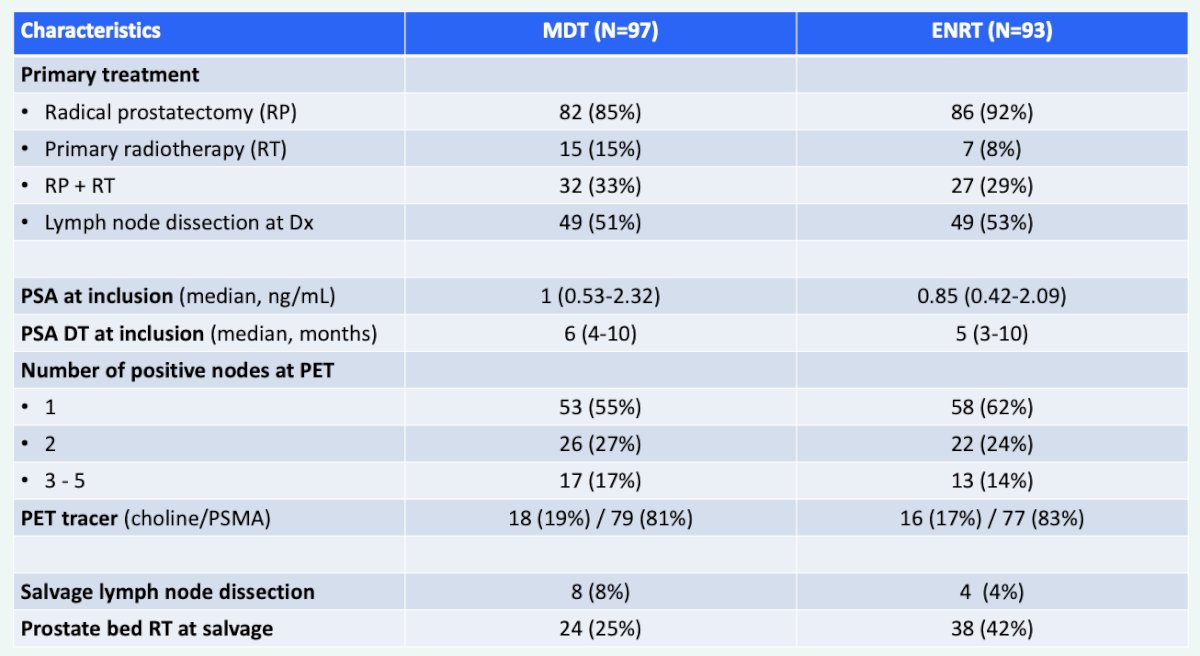
Between June 2018 and April 2021, 196 patients were randomly assigned to metastasis directed therapy or elective nodal pelvic radiotherapy, of which 97 of 99 allocated to metastasis directed therapy and 93 of 97 patients allocated to elective nodal pelvic radiotherapy received per-protocol treatment. The baseline characteristics among those patients receiving treatment are as follows:
Worst late grade ≥ 2 gastrointestinal toxicity was observed in 5.3% of the patients (n = 5, grade 2) in the metastasis directed therapy arm vs 6.6% of the patients (n = 4, grade 2; n = 2, grade 3) in the elective nodal pelvic radiotherapy arm (p = 0.94):

Worst late genitourinary toxicity proportions were as follows: grade 2 and 3 events in 18 (19%) and 3 (3.2%) patients in the metastasis directed therapy arm vs 19 (21%) and 5 (5.5%) patients in the elective nodal pelvic radiotherapy arm (p = 0.61):

Finally, there were no significant differences in the proportion of patients with a clinically meaningful difference in bowel scores between arms for any of the time points during the first 2 years.
Dr. Zilli concluded his presentation by discussing 24-month toxicity results of the PEACE V-STORM trial by emphasizing that elective nodal pelvic radiotherapy and metastasis directed therapy continued to show acceptable toxicity rates during the first 24 months of follow-up. There were no clinically meaningful differences in the occurrence of worst grade ≥ 2 gastrointestinal or genitourinary side effects or decline in bowel quality of life scores compared to baseline.
Presented by: Thomas Zilli, MD, Oncology Institute of Southern Switzerland, Bellinzona, Switzerland
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th – April 8th, 2024


