(UroToday.com) The 2024 European Association of Urology (EAU) annual meeting featured a session on metastatic prostate cancer, and a presentation by Dr. Bertrand Tombal discussing the efficacy and safety of darolutamide in combination with ADT and docetaxel in European patients from the phase 3 ARASENS trial.
Darolutamide is a structurally distinct and highly potent androgen receptor inhibitor with low blood-brain barrier penetration and limited potential for drug-drug interactions. In the ARASENS trial in patients with metastatic hormone-sensitive prostate cancer (mHSPC), darolutamide + ADT + docetaxel significantly reduced the risk of death by 32.5% (HR 0.68; 95% CI 0.57–0.80; p < 0.0001) with similar incidence of treatment-emergent adverse events vs placebo + ADT + docetaxel.1 Darolutamide also improved key patient-relevant secondary endpoints, including time to castration resistant prostate cancer (CRPC), and time to pain progression versus placebo. At the EAU 2024 annual meeting, Dr. Tombal and colleagues reported efficacy and safety results for the subgroup of patients in ARASENS from European countries.
ARASENS is a global, randomized, double-blind, placebo controlled phase 3 study conducted at 286 centers in 23 countries. Patients were randomized to receive darolutamide 600 mg orally twice daily or placebo, with ADT + 6 cycles of docetaxel. Overall survival was the primary endpoint, and key secondary endpoints included time to CRPC, time to pain progression, and safety.
Of 1,305 patients analyzed in ARASENS, the European subgroup was comprised of 472 patients (36.2%; darolutamide, n=240; placebo, n=232):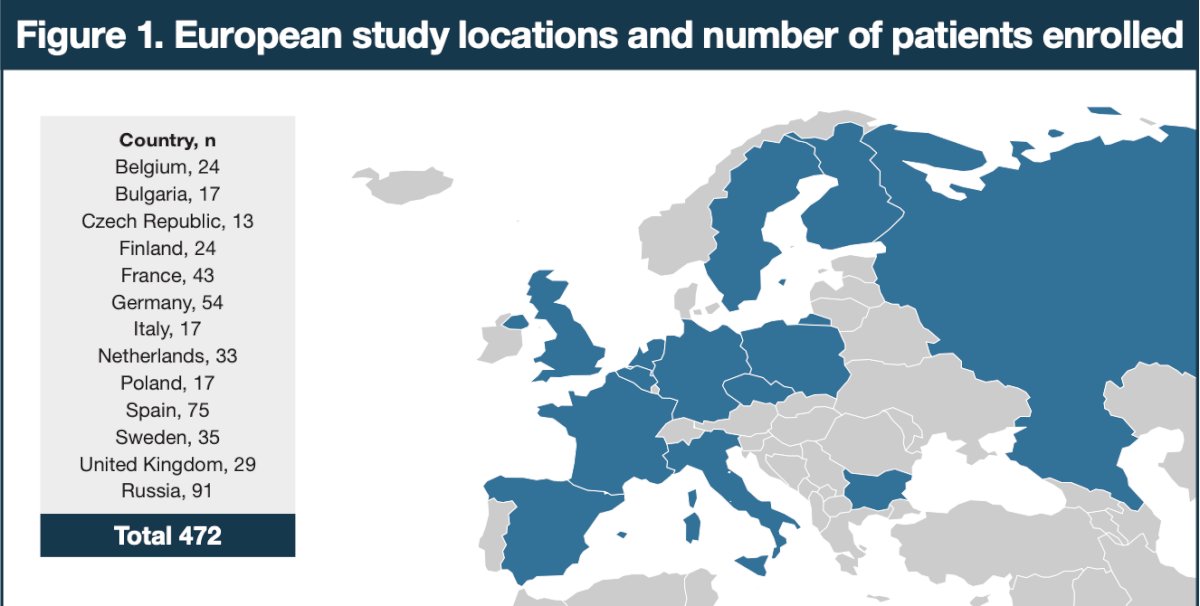
Baseline characteristics of European patients were similar to those of the overall population, with 85% having de novo metastatic disease and 76% having high-volume disease: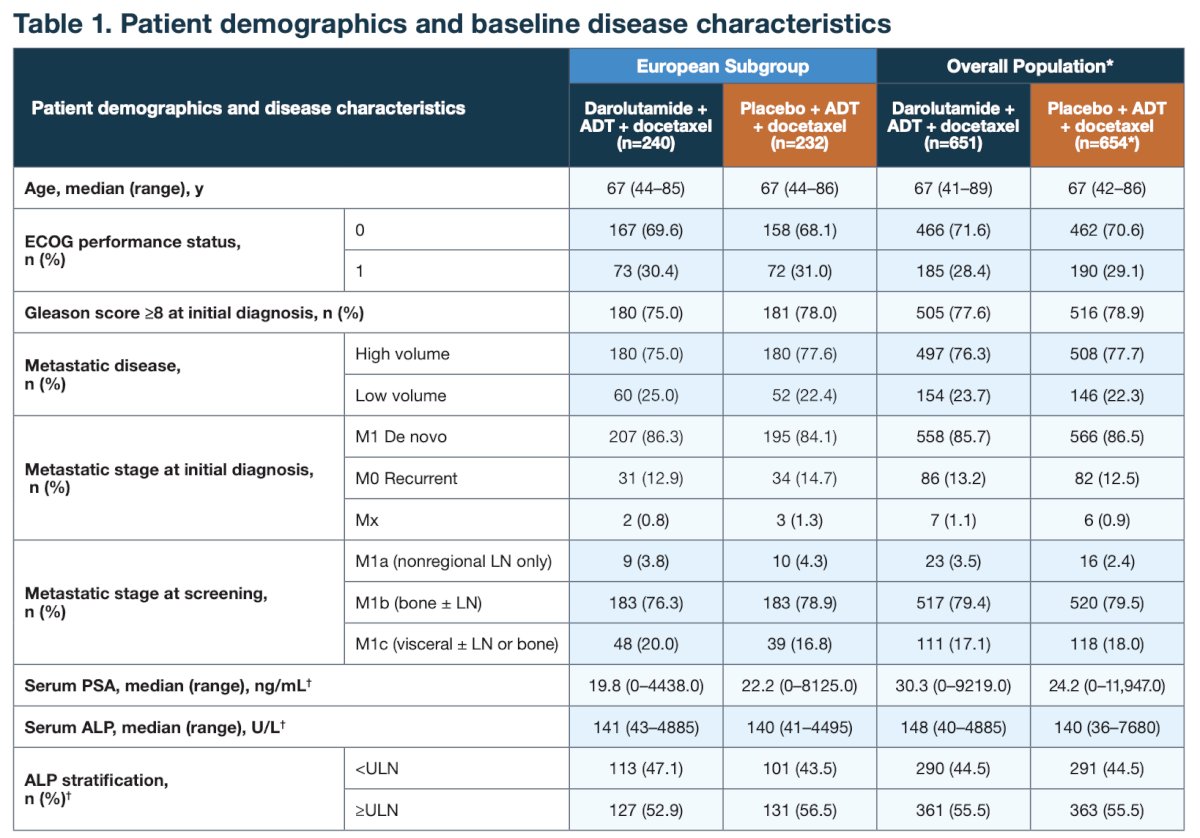
In European patients, darolutamide + ADT + docetaxel increased overall survival, with a 37% reduction in the risk of death vs placebo + ADT + docetaxel (HR 0.63, 95% CI 0.48–0.83), with an improvement in 4-year survival rate (61.6% vs 43.7%), consistent with the overall population:
Compared with the placebo group, the darolutamide group also had longer time to CRPC (HR 0.31, 95% CI 0.17–0.55) versus placebo + ADT + docetaxel in European patients, consistent with the overall population: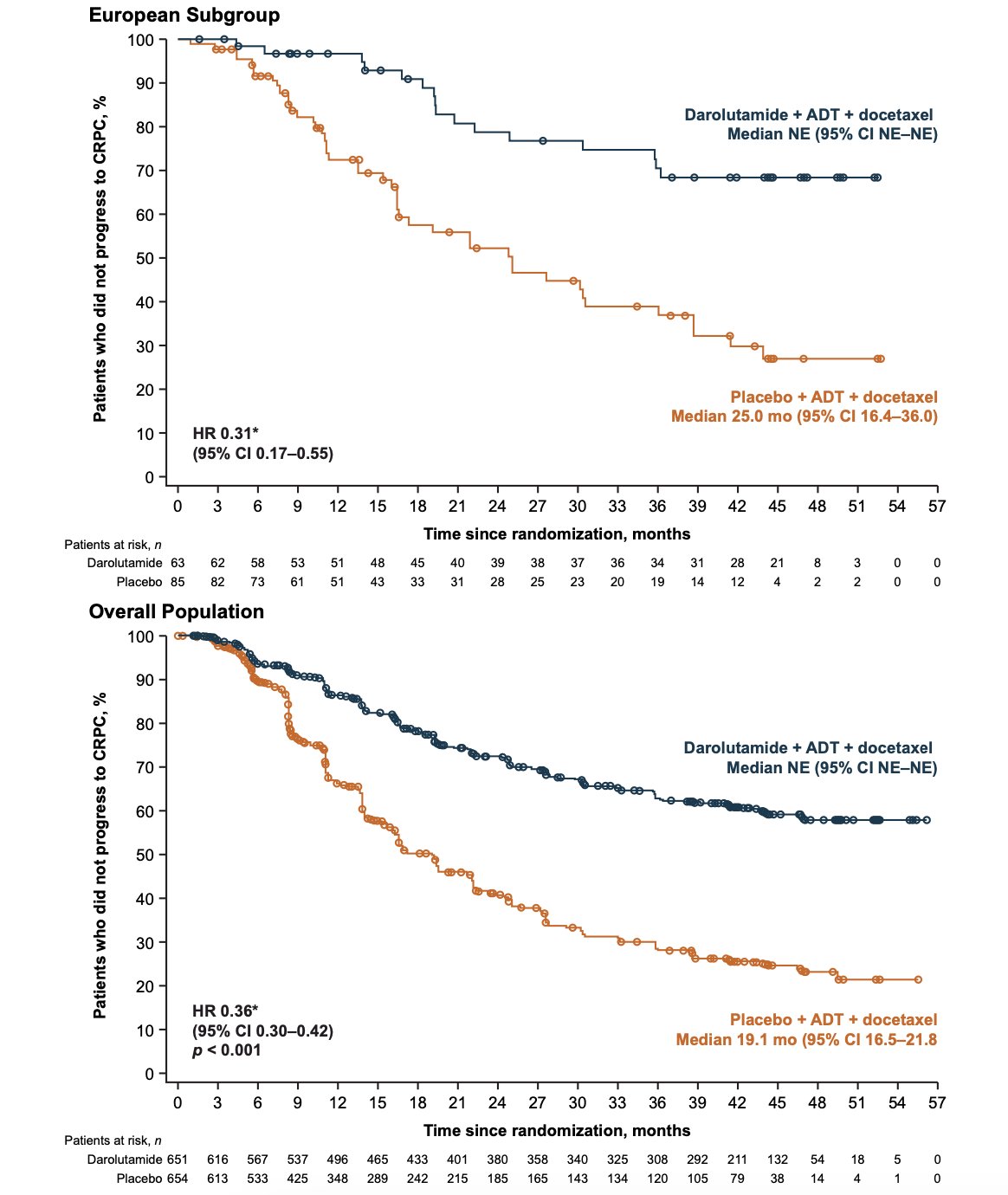
The survival benefit of darolutamide was observed despite a high percentage of placebo patients receiving subsequent life-prolonging systemic therapy in the European subgroup (71.7%), consistent with the overall population: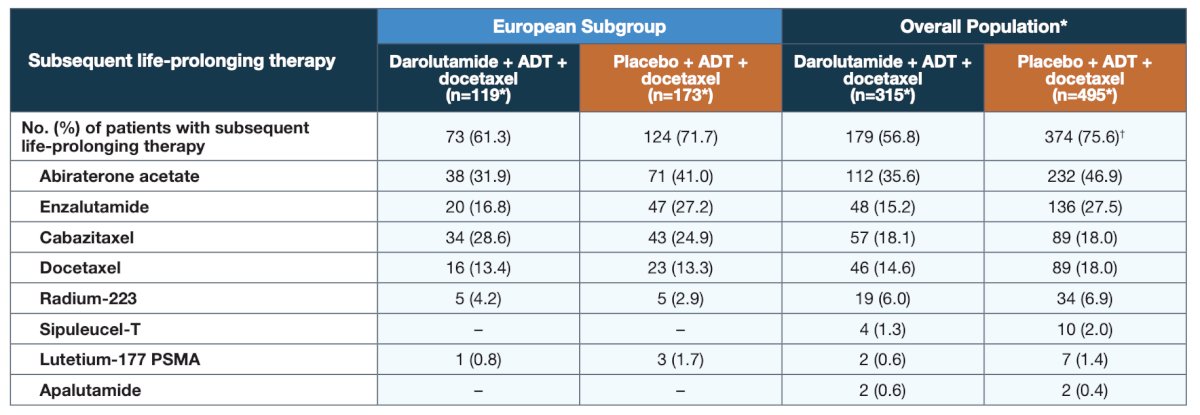
Of note, neither apalutamide nor sipuleucel-T were used as subsequent life-prolonging therapy in the European patients, as neither have regional approval for use in the second line CRPC setting. Darolutamide provided consistent benefits for additional patient-relevant secondary efficacy endpoints versus placebo in European patients: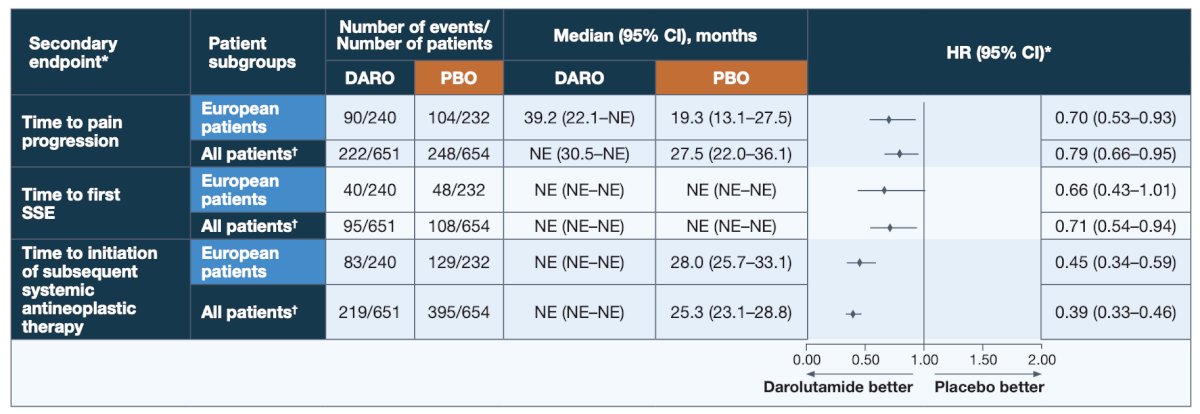
Despite longer treatment duration in darolutamide versus placebo patients (41.4 vs 17.1 months), the incidence of treatment emergent adverse events was similar between treatment groups in the European subgroup, consistent with the overall population: Treatment emergent adverse events commonly associated with androgen receptor pathway inhibitors were generally similar between darolutamide and placebo, particularly when adjusted for treatment exposure:
Treatment emergent adverse events commonly associated with androgen receptor pathway inhibitors were generally similar between darolutamide and placebo, particularly when adjusted for treatment exposure: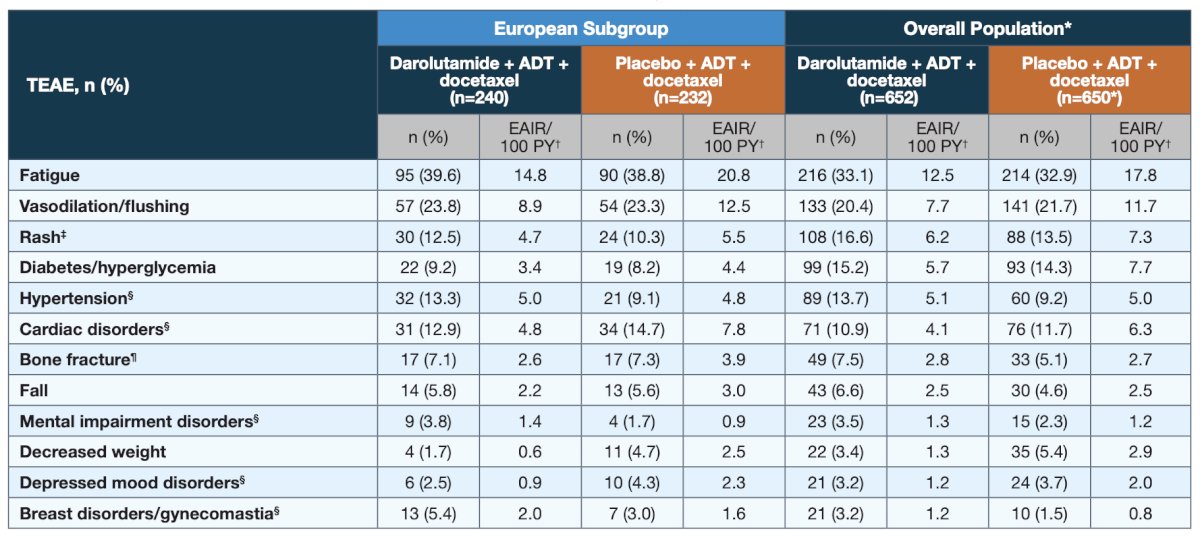
In both the European and overall populations, incidences of the most common grade 3/4 adverse events, most of which are known adverse events related to docetaxel, were highest during the period when patients were receiving docetaxel and either darolutamide or placebo.
Dr. Tombal concluded his presentation discussing the efficacy and safety of darolutamide in combination with ADT and docetaxel in European patients from the phase 3 ARASENS trial with the following statements:
- For European patients in ARASENS, darolutamide improved overall survival and key clinically relevant secondary efficacy endpoints including time to CRPC and time to pain progression
- Darolutamide was well tolerated in this population, with similar incidences of treatment-emergent adverse events compared to placebo
- The efficacy and safety findings in the European population were consistent with the overall ARASENS population
- Darolutamide in combination with ADT and docetaxel should be considered as one of the new standards of care for treatment of patients with mHSPC
Presented by: Bertrand Tombal, MD, PhD, Saint Luc University Clinics, Brussels, Belgium
Written by: Zachary Klaassen, MD, MSc - Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, WellStar MCG Health, @zklaassen_md on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
Related content: Darolutamide Outcomes in European Patients with Metastatic Hormone-Sensitive Prostate Cancer from the ARASENS Trial - Bertrand Tombal
References:


