(UroToday.com) The 2024 European Association of Urology (EAU) annual congress held in Paris, France between April 5th and 8th was host to an abstract session on treatment intensification to improve prostate cancer outcomes. Dr. Alexander Giesen presented the two-years quality of life (QoL) outcomes of the randomized phase II trial ARNEO trial of neoadjuvant degarelix with or without apalutamide prior to radical prostatectomy for high-risk prostate cancer.
The ARNEO trial is a double-blind, placebo-controlled trial, evaluating the role of neoadjuvant degarelix +/- apalutamide for 12 weeks prior to radical prostatectomy in high-risk prostate cancer patients (n=99). The primary study endpoint was minimal residual disease in the surgical specimen. The initial results were published in European Urology in 2023.1 Patients in the intervention arm of degarelix + apalutamide achieved a significantly higher proportion of minimal residual disease, compared to those in the control arm (38% versus 9%; RR: 4.2, 95% CI: 1.5 – 11, p = 0.002).1
However, the side effects and impact of novel hormonal agents on QoL should not be neglected and have not been well studied in the neoadjuvant setting. As such, the objective of this analysis was to compare QoL outcomes of intensified treatment versus ADT in the neoadjuvant setting pre-surgery.
Serial quality of life assessments of these patients were performed using the validated International Consultation on Incontinence Questionnaire-Urinary Incontinence (ICIQ-UI), the International Index of Erectile Function-5 (IIEF-5), and the EORTC Core Quality of Life Questionnaire (QLQ-C30) questionnaires. Questionnaires were collected prior to initiation of neoadjuvant treatment, following twelve weeks of treatment, after surgery, and annually thereafter until three years postoperatively.
All patients in the ARNEO trial had ≥2 years follow-up and answered the questionnaires at the pre-defined time points. For the ICIQ-UI questionnaire (score range: 0 – 21 points; lower scores reflect better results), there were no significant differences at any of the evaluable study time points. The results were as follows:
- At study inclusion: 0 versus 0
- Prior to radical prostatectomy: 0 versus 0
- Post-radical prostatectomy: 10.5 versus 9
- 1-year post-radical prostatectomy: 4 versus 3
- 2 years post-radical prostatectomy: 5 versus 3
Similarly, there were no significant differences in the IIEF-5 score at any of the time points (higher score reflects better results):
- At study inclusion: 7 versus 6
- Prior to radical prostatectomy: 1 versus 1
- Post-radical prostatectomy: 1 versus 1
- 1-year post-radical prostatectomy: 2 versus 2
- 2 years post-radical prostatectomy: 1 versus 2
Similar results were observed for the comparison of median global health scores (QLQ-C30; range: 0 – 100) between the two arms:
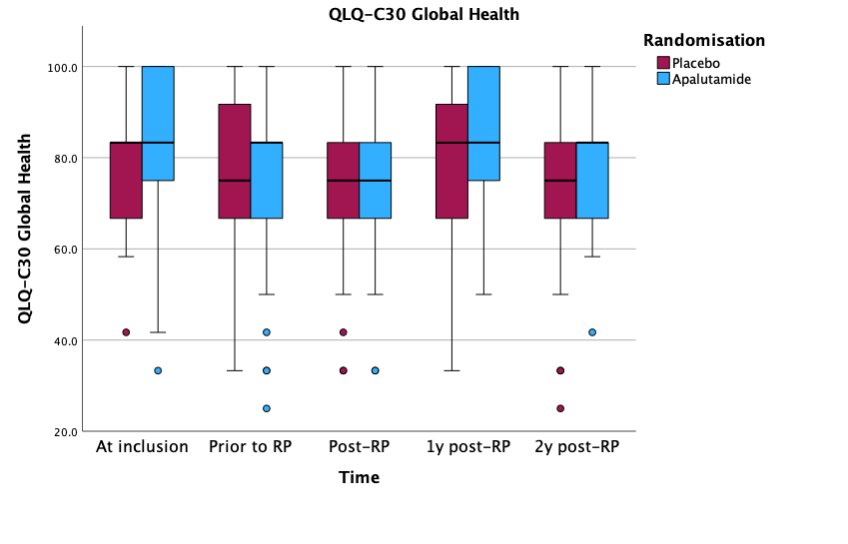
The investigators concluded that in the ARNEO trial, there were no significant differences in quality-of-life outcomes, as assessed using the ICIQ-UI, IIEF-5 and QLQ-C30 questionnaires, between patients receiving degarelix + apalutamide versus degarelix + placebo during two years of follow-up.
Presented by: Alexander Giesen, MD, Urology Resident Physician, Department of Urology, University Hospitals Leuven, Leuven, Belgium
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th – April 8th, 2024References:
- Devos G, Tosco L, Baldewijns M, et al. ARNEO: A Randomized Phase II Trial of Neoadjuvant Degarelix with or Without Apalutamide Prior to Radical Prostatectomy for High-risk Prostate Cancer. Eur Urol. 2023;83(6):508-518.


