(UroToday.com) The 2024 European Association of Urology (EAU) annual congress held in Paris, France between April 5th and 8th was host to an abstract session on treatment intensification to improve prostate cancer outcomes. Dr. Gaëtan Devos presented the three-year oncological outcomes of the randomized phase II ARNEO trial that evaluated neoadjuvant degarelix with or without apalutamide prior to radical prostatectomy for high-risk prostate cancer patients.
Dr. Devos noted that high-risk prostate cancer patients remain at high risk for treatment failure with biochemical recurrence and progression to metastatic disease following single modality local therapy approaches. As such, there has long been interest in evaluating neoadjuvant treatments prior to radical prostatectomy to improve survival outcomes. Such trials to date have been negative, and neoadjuvant therapy is currently not a guideline recommended outside the context of a clinical trial.
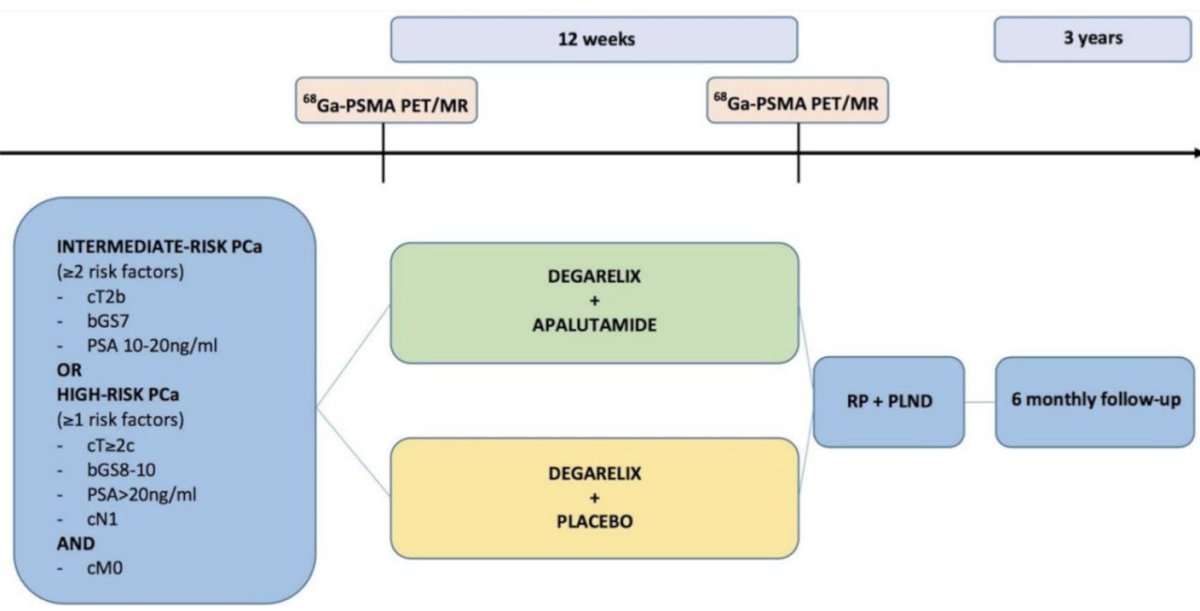
The ARNEO trial is a double-blind, placebo-controlled trial, evaluating the role of neoadjuvant degarelix +/- apalutamide prior to radical prostatectomy in high-risk prostate cancer patients (n=99). The primary study endpoint was minimal residual disease in the surgical specimen. The initial results were published in European Urology in 2023.1 Patients in the intervention arm of degarelix + apalutamide achieved a significantly higher proportion of minimal residual disease, compared to those in the control arm (38% versus 9%; RR: 4.2, 95% CI: 1.5 – 11, p = 0.002).1 However, it remains unclear if these improved pathological outcomes correlate with improved oncological outcomes. Moreover, the investigators did not include a control arm that received the current standard of care (i.e., surgery without neoadjuvant therapy) in this trial.
Patients were followed up with PSA and testosterone testing every 6 months. No adjuvant radiotherapy or hormonal therapy was given. At the time of PSA relapse, all patients underwent a PSMA-PET/CT. In patients with negative imaging, salvage radiotherapy to the prostate bed (+/- pelvic region) was recommended. Using propensity score matching, the investigators matched patients from the degarelix + apalutamide arm of the ARNEO trial to patients treated with surgery alone in their hospital during the same period and included those as a ‘standard of care’ control arm in subsequent survival analyses.
The median follow-up was 34 months in the intervention arm of degarelix + apalutamide and 32 months in the degarelix + placebo arm. Biochemical recurrence occurred in 22% and 36% of patients in the degarelix + apalutamide and degarelix + placebo arms, respectively. There was a non-significant 42% decrease in the rate of biochemical recurrence with the addition of apalutamide (HR: 0.58, 95% CI: 0.27 – 1.25, p=0.14):

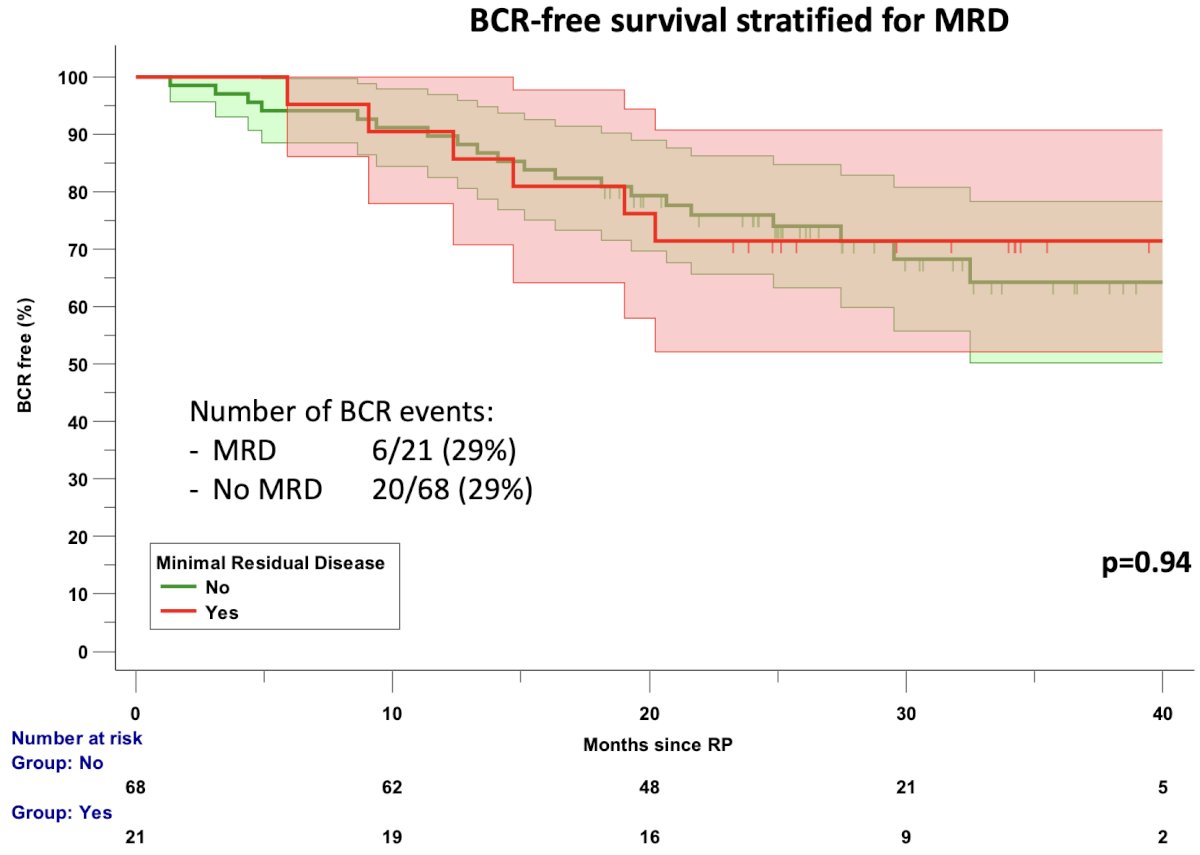
When biochemical recurrence-free survival was stratified by minimal residual disease (yes/no), there was no significant difference in outcomes between those who achieved minimal residual disease (primary study endpoint) and those who did not. This results brings into question the clinical relevance of the primary study outcome choice.
When biochemical recurrence-free survival was stratified by pathologic stage, as expected, survival outcomes were superior in those with ypT2 disease (versus ypT3-4).

Metastatic disease spread was noted for one patient (2.2%) in the degarelix + apalutamide arm, compared to six patients (14%) in the degarelix + placebo arm (p=0.04).
There was a median follow-up of 36 months (IQR: 30 – 44 months) in the matched standard of care arm. No patients in the matched degarelix + apalutamide arm (n=38) developed metastatic disease, compared to 4 patients (11%) in the standard of care arm (n=38; p=0.04).
Dr. Devos concluded that:
- At 3 years follow-up, there was no statistically significant differences in biochemical recurrence between patients treated with neoadjuvant degarelix + apalutamide versus degarelix + placebo (HR: 0.58, p=0.14)
- The primary study endpoint of minimal residual disease was not associated with improved biochemical recurrence-free survival. However, organ confined disease (i.e., ypT2) following neoadjuvant hormonal therapy was significantly associated with improved biochemical recurrence-free survival, compared to ypT3-4 disease.
- Patients treated with neoadjuvant degarelix + apalutamide had improved metastatic disease-free survival compared to both degarelix alone and the matched standard of care cohort of radical prostatectomy alone.
Presented by: Gaëtan Devos, Resident Physician, University Hospitals Leuven, Department of Urology, Leuven, Belgium
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th – April 8th, 2024
References:
- Devos G, Tosco L, Baldewijns M, et al. ARNEO: A Randomized Phase II Trial of Neoadjuvant Degarelix with or Without Apalutamide Prior to Radical Prostatectomy for High-risk Prostate Cancer. Eur Urol. 2023;83(6):508-518.


