(UroToday.com) The 2024 European Association of Urology (EAU) annual congress held in Paris, France between April 5th and 8th was host to an abstract session on treatment intensification to improve prostate cancer outcomes. Dr. Colin Belliveau presented the results of a phase II randomized controlled trial evaluating prostate-specific membrane antigen (PSMA)-positron emission tomography (PET) guided intensification of salvage radiotherapy following radical prostatectomy.
Dr. Belliveau noted that early salvage radiation therapy following radical prostatectomy is currently considered a standard of care approach for the treatment of patients with biochemical failure. PSMA-PET/CT has been widely adopted in this setting, owing to its improved performance characteristics compared to conventional imaging,1 and has been shown to influence treatment decision-making.2,3 However, to date, it remains unknown whether such changes in management are associated with improved outcomes. The primary objective of this study was to determine if PSMA-PET guided intensification of salvage radiotherapy following radical prostatectomy improves failure-free survival outcomes.
This was a phase II, multicenter randomized controlled trial (NCT03525288) which included patients planned for definitive radiotherapy, and who were enrolled between May 2018 and February 2021 across four groups. In this study, the investigators reported on the group consisting of 128 subjects with biochemical failure (definition: PSA > 0.1 ng/mL) following prostatectomy and were planned for salvage radiotherapy.
Patients randomized to the experimental arm underwent an 18F-DCFPyL PSMA-PET/CT prior to treatment, and radiotherapy was subsequently intensified targeting newly identified disease sites identified on PSMA-PET/CT, per the protocol guidelines. Interim analysis of the primary failure-free survival endpoint for the salvage post-prostatectomy cohort was triggered by the number of failure events reported. In this population, biochemical recurrence was defined as PSA nadir + 0.2 ng/mL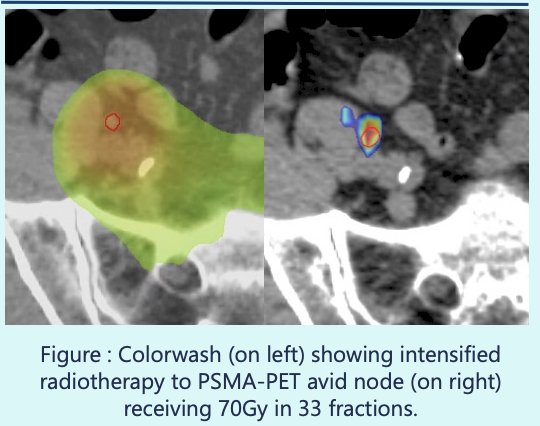
The median PSA at study entry was 0.3 ng/ml (range: 0.1–3 ng/ml). ‘Intensified’ salvage radiotherapy was delivered to 33/64 subjects who underwent PSMA-PET/CT:
- Addition of pelvic salvage radiotherapy (n=16)
- Addition of metastases-directed radiotherapy (n=2)
- Lymph node boost (n=19)
- Prostate bed boost (n=15)
The use of adjuvant hormone therapy was equally high in both arms (86% in the control arm versus 84% in the PSMA-PET/CT imaged arm). At a median follow-up of 37 months, use of PSMA-PET/CT-guided intensification significantly improved failure-free survival outcomes (HR: 0.46, 95% CI: 0.21 – 0.98, p=0.04; 20 versus 10 events). To date, there are no differences in metastasis-free survival based on conventional imaging and in overall survival (one death in each arm).
The investigators concluded that this randomized trial demonstrates improved prostate cancer control outcomes with PSMA-PET/CT-guided intensification of salvage radiotherapy post-radical prostatectomy. They argued that patients planned for salvage radiotherapy following radical prostatectomy should be prioritized for access given its impact on outcomes, particularly given the limitations of PSMA-PET/CT access across numerous healthcare systems. A phase III trial (NCT04557501) has completed accrual and will shed further light on those patients who benefit most from PSMA-PET/CT-guided intensification of salvage radiotherapy.
Presented by: Colin Belliveau, MD, Resident Physician, Département de radio-oncologie, Centre Hospitalier de l'Université de Montréal (CHUM), Montréal, Québec, Canada.
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
References:
- Hofman MS, Lawrentschuk N, Francis, RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomized, multicentre study. Lancet 2020 Apr 11;395(10231):1208-1216.
- Morris MJ, Rowe SP, Gorin MA, et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin Cancer Res. 2021 Jul 1;27(13):3674-3682.
- Pienta KJ, Gorin MA, Rowe SP, et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in Prostate Cancer Patients (OSPREY). J Urol. 2021 Jul;206(1):52-61.


