(UroToday.com) The 2024 European Association of Urology (EAU) annual meeting featured a plenary session on personalized approaches in high-risk and metastatic prostate cancer, and a presentation by Dr. Maria De Santis discussing the guideline’s view for the utilization of PARP inhibitors in patients with alterations in DNA repair genes. Dr. De Santis starting by emphasizing that the guidelines state that “All metastatic patients should be offered somatic genomic testing for homologous repair and MMR defects early on, preferably before first line mCRPC treatment is established.”
Dr. De Santis provided a summary of evidence and the EAU guidelines for life-prolonging treatment of castrate-resistant prostate cancer:
- Ensure that testosterone levels are confirmed to be < 50 ng/dL before diagnosing castrate-resistant prostate cancer (Strength of recommendation: Strong)
- Counsel, manage, and treat patients with mCRPC in a multidisciplinary team (Strength of recommendation: Strong)
- Treat patients with mCRPC with life-prolonging agents (Strength of recommendation: Strong)
- Offer mCRPC patients somatic and/or germline molecular testing, as well as testing for mismatch repair deficiencies or microsatellite instability (Strength of recommendation: Strong)
The EAU guidelines have a specific statement for mCRPC first line combination therapy: “The combination of ARPI + PARP inhibitors showed a significant rPFS benefit in RCTs for unselected patients. However, this benefit is mainly driven by HRR-altered and even more pronounced by BRCA1/2-altered patients. So far, no clear overall survival benefit has been seen, and the side effects of PARP inhibitors add substantial toxicity to ARPI monotherapy. Therefore, no recommendation is given for patients without HRR and BRCA 1/2-mutations and the data will be re-evaluated after longer follow-up.”
In an FDA approval summary of PROpel,1 Fallah et al.2 emphasized that in patients with mCRPC, efficacy of the combination of olaparib plus abiraterone was primarily attributed to the treatment effect in the BRCA mutated subgroup, the indicated population for the approval. For patients without a BRCA mutation, the FDA determined that the modest rPFS improvement, combined with clinically significant toxicities, did not demonstrate a favorable risk/benefit assessment. The Kaplan-Meier plots from PROpel of three BRCA-based subgroups are as follows:
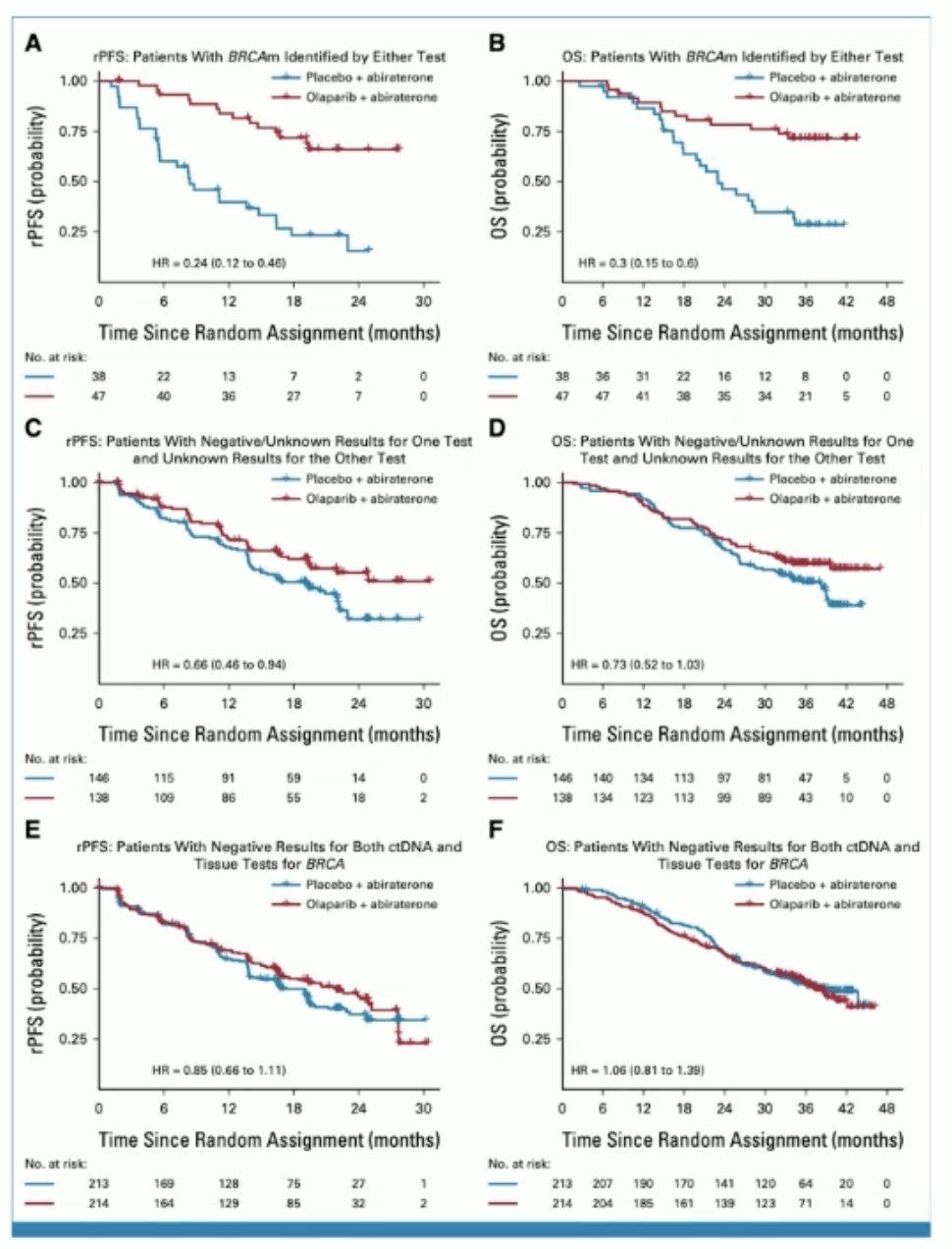
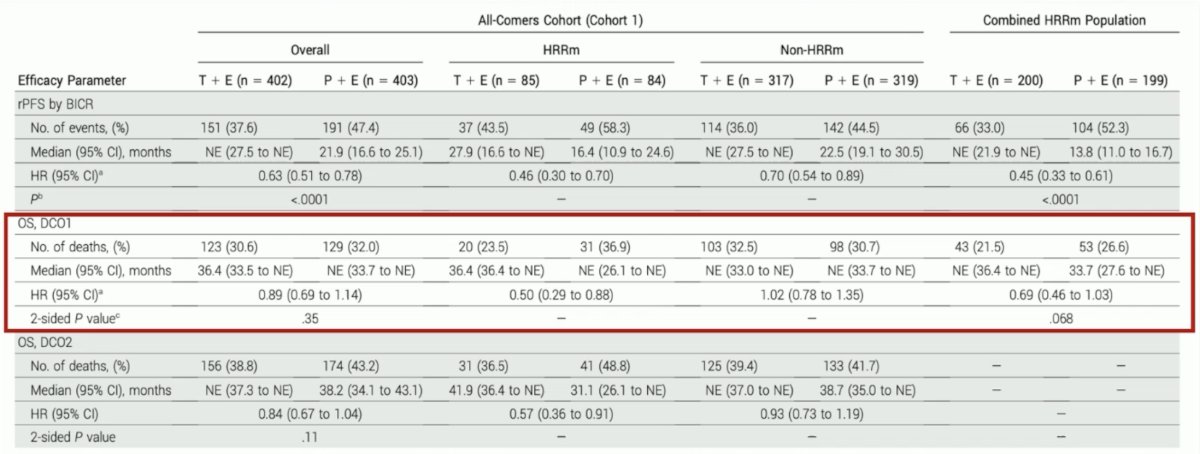
In an FDA approval summary of TALAPRO-2,3 Heiss et al.4 emphasized that despite a statistically significant rPFS improvement in the all-comer cohort, the FDA did not consider the magnitude of rPFS clinically meaningful in the context of the broad indication, combination treatment, and safety profile. Approval was therefore limited to patients with HRR mutated mCRPC, for whom there was a statistically significant and clinically meaningful improvement in rPFS and favorable overall survival results:
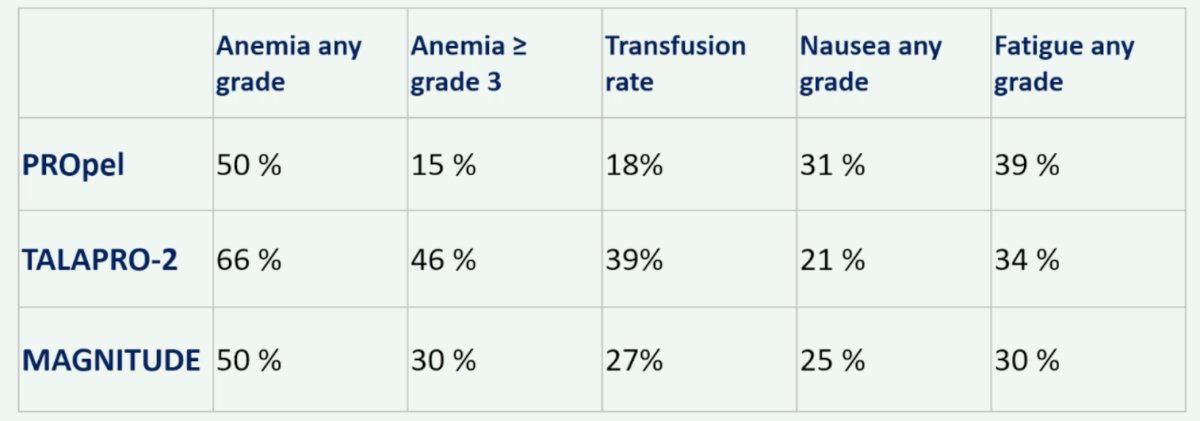
Finally, Dr. De Santis summarized the side effects of ARPI + PARP inhibitor combinations in the following table:
Dr. De Santis concluded her presentation by discussing the guideline’s view for the utilization of PARP inhibitors in patients with alterations in DNA repair genes with the following conclusions regarding the recommendations for the use of PARP inhibitors in first line mCRPC:
- Offer patients previously untreated for mCRPC and harboring an HRR or BRCA mutation abiraterone in combination with olaparib if the patient is fit for both agents (Strength of recommendation: Strong)
- Offer patients previously untreated for mCRPC and harboring a BRCA mutation abiraterone in combination with niraparib if the patient is fit for both agents (Strength of recommendation: Strong)
- Offer patients previously untreated for mCRPC and harboring an HRR-mutation enzalutamide in combination with talazoparib if the patient is fit for both agents (Strength of recommendation: Strong)
Presented by: Maria De Santis, MD, PhD, Charité Universitatsmedizin, Berlin, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th – April 8th, 2024
References:
- Saad F, Clarke NW, Oya M, et al. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): final prespecified overall survival results of a randomized, double-blind, phase 3 trial. Lancet Oncol. 2023 Oct;24(10):1094-1108.
- Fallah J, Xu J, Weinstock C, et al. FDA Approval Summary: Olaparib in combination with Abiraterone for Treatment of patients with BRCA-mutated Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. 2024 Feb 10;42(5):605-613.
- Agarwal N, Azad AA, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): A randomized, placebo-controlled, phase 3 trial. Lancet. 2023 Jul 22;402(10398):291-303.
- Heiss BL, Chang E, Gao X, et al. US Food and Drug Administration Approval Summary: Talazoparib in Combination with Enzalutamide for Treatment of Patients with Homologous Recombination Repair Gene-Mutated Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. 2024 Mar [Epub ahead of print].


