The 2024 European Association of Urology (EAU) annual congress held in Paris, France between April 5th and 8th was host to a joint session of the EAU and the Advanced Prostate Cancer Consensus (APCCC). Professor Thomas Zilli discussed the evidence for radiotherapy for the treatment of patients with synchronous, low-volume metastatic hormone sensitive prostate cancer (mHSPC).
Professor Zilli noted that the two main ‘open questions’ for the radiation oncologist in this setting remain:
- Should we treat the primary disease site?
- Is there a rationale for including all disease sites?
To date, there are three randomized trials that have evaluated the role of prostate radiotherapy for patients with de novo, low volume mHSPC: HORRAD, STAMPEDE (Arm H), and PEACE-1.
HORRAD was a multicenter, prospective, randomized controlled trial of 432 patients with previously untreated, de novo mHSPC at 28 centers across The Netherlands between November 2004 and September 2014. Patients were randomized in a 1:1 fashion to either ADT with external beam radiotherapy (EBRT) or ADT alone. At a median follow up of 47 months, there were no significant differences in median overall survival between the two treatment arms: 45 and 43 months in the EBRT + ADT and ADT arms, respectively (HR: 0.90, 95% CI: 0.70-1.14, p=0.4). However, subgroup analysis by number of metastatic lesions suggested a potential overall survival benefit for radiotherapy in patients with <5 metastatic sites (HR: 0.68, 95% CI: 0.42-1.10).1
STAMPEDE (Arm H) was an open label, randomized controlled phase III trial of 2,061 men from 117 hospitals across Switzerland and the UK. This arm randomized patients with de novo mHSPC in a 1:1 fashion to standard of care plus radiotherapy versus standard of care alone between January 2013 and September 2016. Standard of care was lifelong ADT with upfront docetaxel permitted from December 2015. Men allocated to radiotherapy received either a daily (55 Gy in 20 fractions over 4 weeks) or weekly (36 Gy in six fractions over 6 weeks) schedule that was nominated before randomization. In the overall cohort, radiotherapy improved failure-free survival (HR 0.76, 95% CI 0.68–0.84) but not overall survival (HR 0.92, 95% CI 0.80–1.06). However, when stratified by metastatic burden, overall survival benefits were seen in the CHAARTED low volume group (HR 0.68, 95% CI 0.52-0.90) with restricted mean survival time improved by 3.6 months from 45.4 to 49.1.2 Updated results of this trial were published in 2022. With a median follow up of 61.3 months, prostate radiotherapy continued to demonstrate overall survival benefits in patients with low metastatic burden (HR 0.64, 95% CI 0.52-0.79).3
But can we further delineate which low-volume patients may benefit most from prostate radiotherapy? A follow-up analysis by Ali et al. published in 2021 demonstrated that the overall survival benefit with prostate radiotherapy for de novo, low volume mHSPC appears to be greatest in patients with only non-regional lymph nodes (M1a) or ≤3 bone metastases without visceral metastases (HR: 0.63, 95% CI: 0.46 – 0.83).4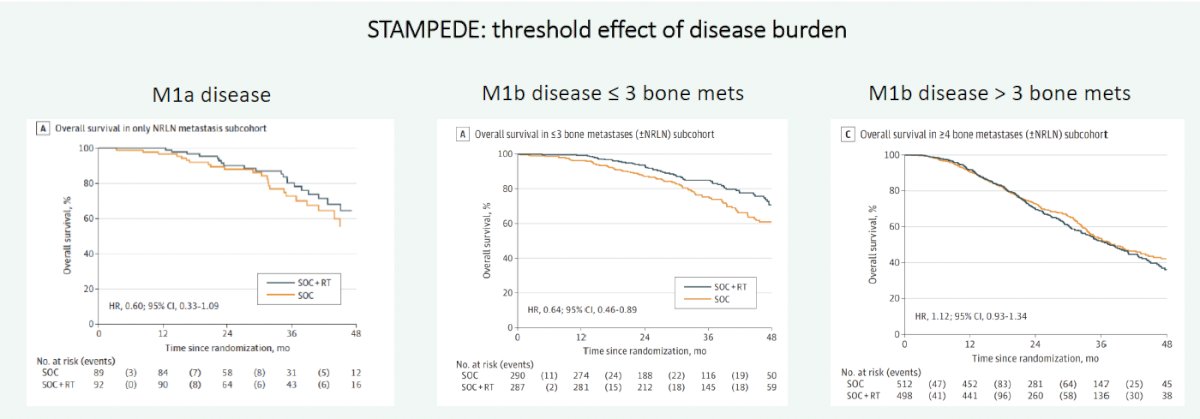
Based on these results, the EAU guidelines currently ‘strongly’ recommend offering ‘ADT combined with non-curative prostate radiotherapy (using doses up to the equivalent of 72 Gy in 2 Gy fractions) to patients whose first presentation is M1 disease and who have low volume of disease by CHAARTED criteria/M1a disease”.
In contrast to the HORRAD and STAMPEDE Arm H trials which evaluated the addition of prostate radiotherapy to ADT (concurrent docetaxel: 18% of STAMPEDE patients, none in HORRAD; androgen receptor pathway inhibitor: none in both trials), the PEACE-1 trial evaluated the role of prostate radiotherapy in the setting of treatment intensification with docetaxel and/or abiraterone acetate. Presented at ASCO 2023, PEACE-1 employed a two-by-two factorial design to assess, both separately and combined, the impact of abiraterone acetate/prednisone and prostate radiotherapy addition to standard of care therapy in men with de novo mHSPC. This trial randomized 1,173 patients in a 1:1:1:1 fashion to standard of care +/- abiraterone +/- radiotherapy:
Standard of care treatment included continuous ADT or bilateral orchiectomy, with or without docetaxel. Various amendments were implemented during the trial due to the evolving standard of care in this disease space. After 2015, docetaxel was permitted as part of the standard of care per investigator’s discretion and patient consent. After CHAARTED and STAMPEDE were reported,5,6 it became unethical to administer ADT alone, and so docetaxel administration was made mandatory.
With regards to overall survival, the addition of prostate radiotherapy to either standard of care alone or standard of care + abiraterone was not associated with significant improvements.
The addition of prostate radiotherapy to standard of care + abiraterone acetate was associated with a significant radiographic progression-free survival (rPFS) benefit (median 7.5 versus 4.4 years, p=0.02). However, the addition of radiotherapy to standard of care alone was not associated with an rPFS benefit (median 2.6 versus 3.0 years; HR 1.11, 95% CI 0.67 – 1.84):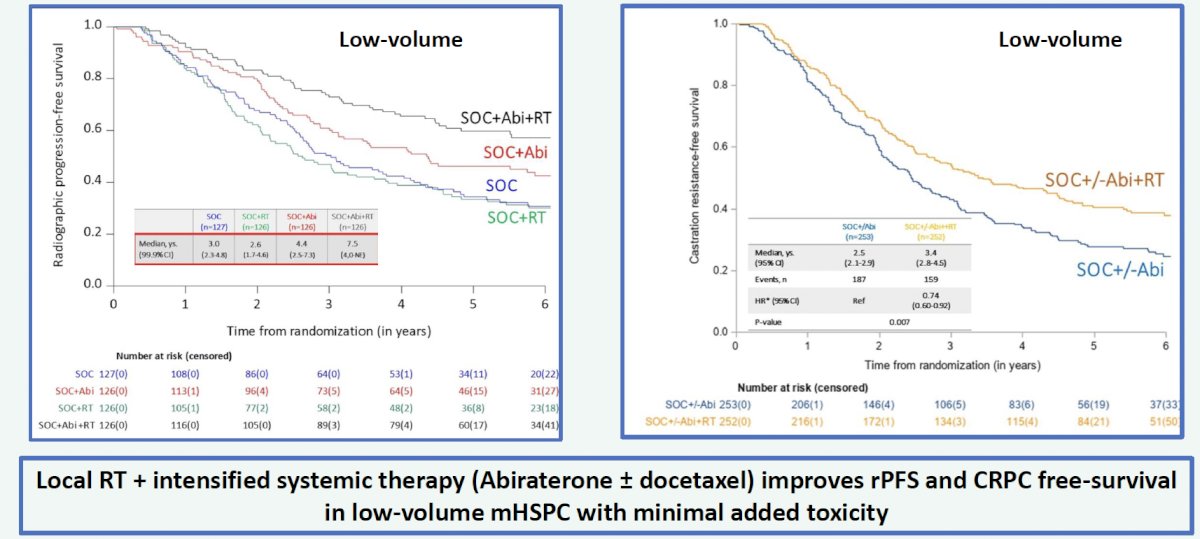
One of the common concerns regarding early treatment intensification is the additive toxicity and potential adverse effects on patient quality of life. Interestingly, the addition of prostate radiotherapy to standard of care +/- abiraterone was associated with significant improvements in time to serious genitourinary events in both the low- and high-volume cohort.
What is the current evidence of treating all metastatic sites (i.e., ‘comprehensive’ radiotherapy?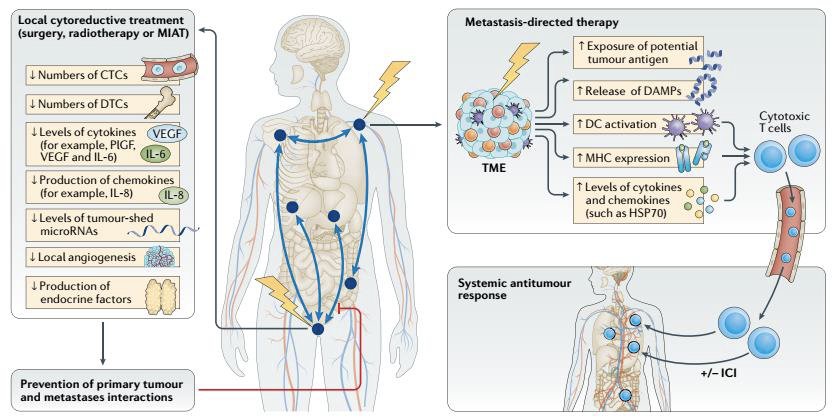
A series of 47 men with de novo oligometastatic prostate cancer from MSKCC were treated with local radiotherapy, ADT, and metastasis-directed therapy to all metastatic sites (≤6 sites). 70% of patients achieved undetectable PSA levels following testosterone recovery.7 A series of 12 patients with de novo oligometastatic prostate cancer (≤5 lesions) from Johns Hopkins were treated with local radiotherapy, chemotherapy, ADT, and SBRT to all metastatic sites. 67% of patients achieved undetectable PSA levels following testosterone recovery at 3 years. The San Raffaele experience of 39 men with de novo oligometastatic prostate cancer treated with local radiotherapy, ADT, and SBRT to all metastatic sites demonstrated that at 4 years, 47% of patients had disease recurrence, 27% developed novel metastatic sites, and 35% progressed to castration resistance.9 Based on this early evidence, Professor Zilli concluded that loco-regional radiotherapy with SBRT to all PET-positive metastatic sites is feasible with promising disease control rates.
Prospective evidence to evaluate the role of ‘comprehensive’ ADT is now available from the SOLAR phase 2 trial of systemic plus tumor-directed therapy for de novo oligometastatic prostate cancer (n=28) between 2018 and 2022 (unpublished – in press at time of presentation). Patients were staged using PSMA (89%), fluciclovine (3.5%), and NaF PET scans, respectively. 29% of patients had M1a disease and 71% had M1b disease. N1 disease was present in 64% of patients. 61% of patients had Grade Group 4–5 disease. The number of metastatic sites was as follows:
- 1: 42%
- 2: 21%
- 3–5: 38%
Treatment consisted of ADT + abiraterone + apalutamide for 6 months followed by a radical prostatectomy plus lymph node dissection versus whole pelvic radiotherapy + MDT to all metastatic sites. The primary endpoint was testosterone recovery and controlled PSA at 6 months following recovery, defined as PSA <0.2 ng/ml following radical prostatectomy and <2 ng/ml following radiotherapy.
83% of patients were free of progression at a median of 31 months. Based on these results, it appears that loco-regional treatment with SBRT to all PET+ metastatic sites + 6 months of intensified systemic therapy achieves lasting remission without ongoing castration in de-novo oligometastatic mHSPC.
Professor Zilli highlighted ongoing clinical trials evaluating the role of ‘comprehensive’ radiotherapy in the metastatic setting:
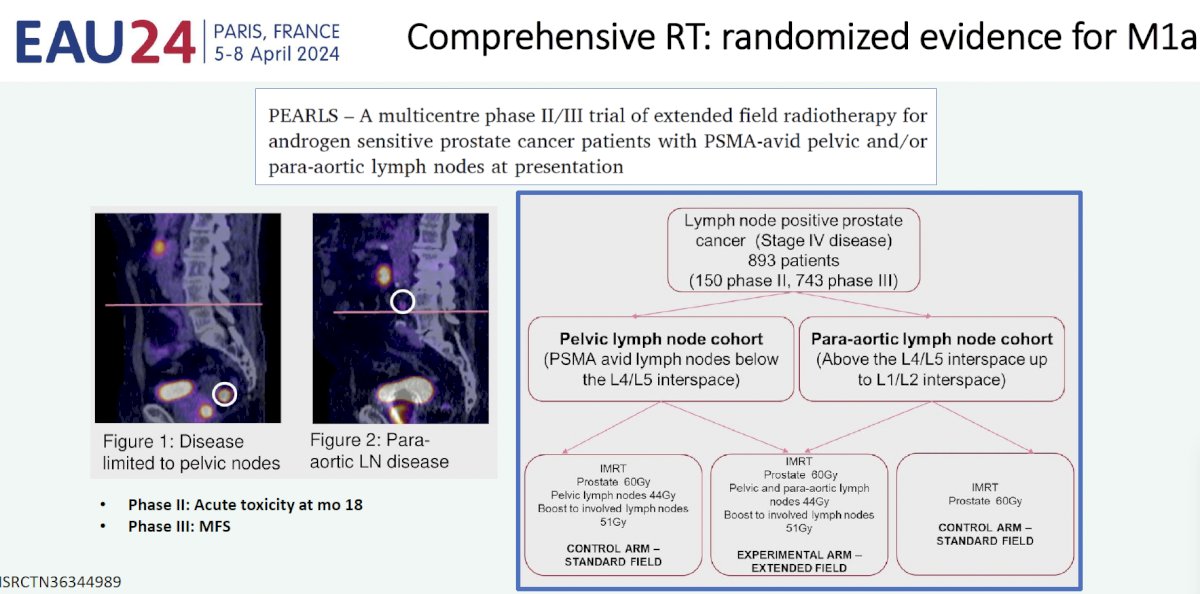
Despite this emerging evidence, for the time being, the APCCC currently recommends that MDT only be offered to patients within a clinical trial setting or a well-designed prospective cohort study (strong recommendation).
Professor Zilli’s take home messages were as follows:
- There is a proven overall survival benefit for prostate radiotherapy in patients with low-volume mHSPC treated with ADT, with or without docetaxel
- There is a role for prostate radiotherapy in preventing rPFS, mCRPC, and serious GU events in low-volume mHSPC patients treated with intensified systemic treatment (androgen receptor pathway inhibitor + standard of care) without increasing toxicity or deteriorating quality of life
- Ongoing RCTs in the de novo oligometastatic mHSPC setting staged per PSMA PET/CT will help define the role of:
- Systemic therapies combined with radiotherapy (type and duration)
- Ablative metastasis directed-therapy (MDT) to all disease sites
- For comprehensive irradiation, consider multidisciplinary discussion and inclusion in clinical trials or registries
Presented by: Professor Thomas Zilli, MD, Radiation Oncology Department, Geneva University Hospital, Geneva, Switzerland
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
References:- Boeve LMS, Hulshof MCCM, Vis AN, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol. 2019;75(3):410-418.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the prostate for men with metastatic prostate cancer in the UK and Switzerland: Long-term results from the STAMPEDE randomised controlled trial. PLoS Medicine. 2022;19(6):e1003998.
- Ali A, Hoyle A, Haran AM, et al. Association of Bone Metastatic Burden With Survival Benefit From Prostate Radiotherapy in Patients With Newly Diagnosed Metastatic Prostate Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2021;7(4): 555-563.
- Sweeney CJ, Chen Y, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015;373:737-746.
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177.
- Imber BS, Varghese M, Goldman DA, et al. Clinical Outcomes of Combined Prostate- and Metastasis-Directed Radiation Therapy for the Treatment of De Novo Oligometastatic Prostate Cancer. Adv Radiat Oncol. 2020;5(6): 1213-1224.
- Reyes DK, Rowe SP, Schaeffer EM, et al. Multidisciplinary total eradication therapy (TET) in men with newly diagnosed oligometastatic prostate cancer. Med Oncol. 2020;37(7):60.
- Deantomi CL, Fodor A, Cozzarini C, et al. Prostate cancer with low burden skeletal disease at diagnosis: outcome of concomitant radiotherapy on primary tumor and metastases. Br J Radiol. 2020;93(1108):20190353.


