(UroToday.com) The 2024 European Association of Urology (EAU) annual congress held in Paris, France between April 5th and 8th was host to a game changing session addressing the best technique for prostate biopsy. Dr. Badar Mian presented the results of the ProBE-PC randomized clinical trial evaluating the efficacy of transrectal and transperineal prostate biopsy for detecting clinically significant prostate cancer.
To date, there has been a paucity of level 1 evidence comparing transperineal and transrectal prostate biopsies with regard to their diagnostic performance and complication/infectious rates. Accordingly, Dr. Mian and colleagues registered the ProBE-PC study (Prostate Biopsy: Efficacy and Complications) in May 2019 (NCT04081636). Prostate biopsies in this trial were performed in an office setting using local anesthesia. The URONAV fusion platform was used for both biopsy techniques. This trial was adequately powered to evaluate the superiority of transperineal biopsies for infectious complications and detection of clinically significant prostate cancer (i.e., Grade Group ≥2).
Initial results of this trial have been published in The Journal of Urology in 2024 and demonstrated no difference in infectious complications, whether assessed as a composite outcome or individually.1
In this presentation, Dr. Mian focused on the cancer detection outcomes. This trial included all men undergoing prostate biopsy in the initial or repeat setting, with or without positive MRI lesions (i.e., PIRADS 3–5). Exclusion criteria included technical considerations, including no access to the rectum.
The target sample size was 840 men.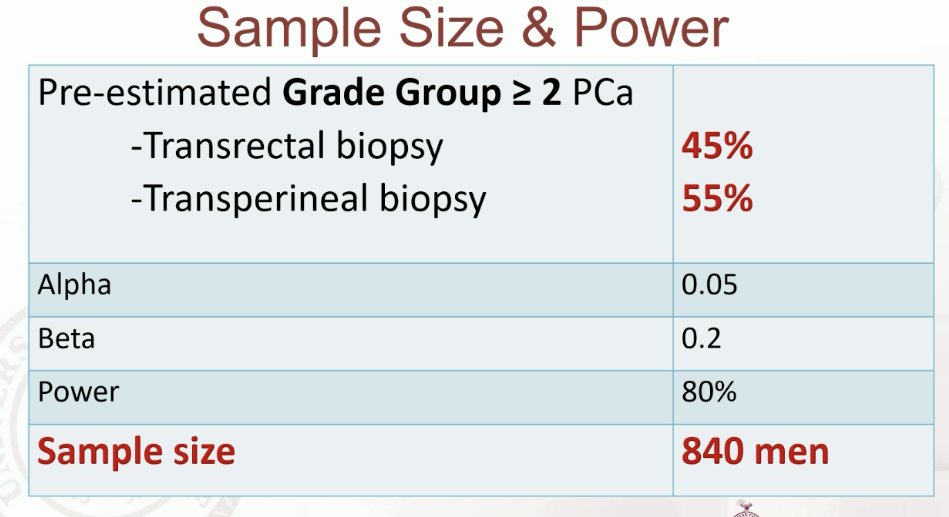
With regards to procedural details, for patients with concerning MRI lesions, a targeted plus systematic biopsy was performed (3 cores per target lesion). A 12-core systematic biopsy was performed in the absence of an MRI. With regards to baseline characteristics, the median patient age was 65–66 years of age. 96% of patients had a pre-biopsy mpMRI performed, and 72% had a PIRADS 4–5 lesion. Anterior lesions were present in 33% and 31% of patients in the transrectal and transperineal biopsy groups, respectively.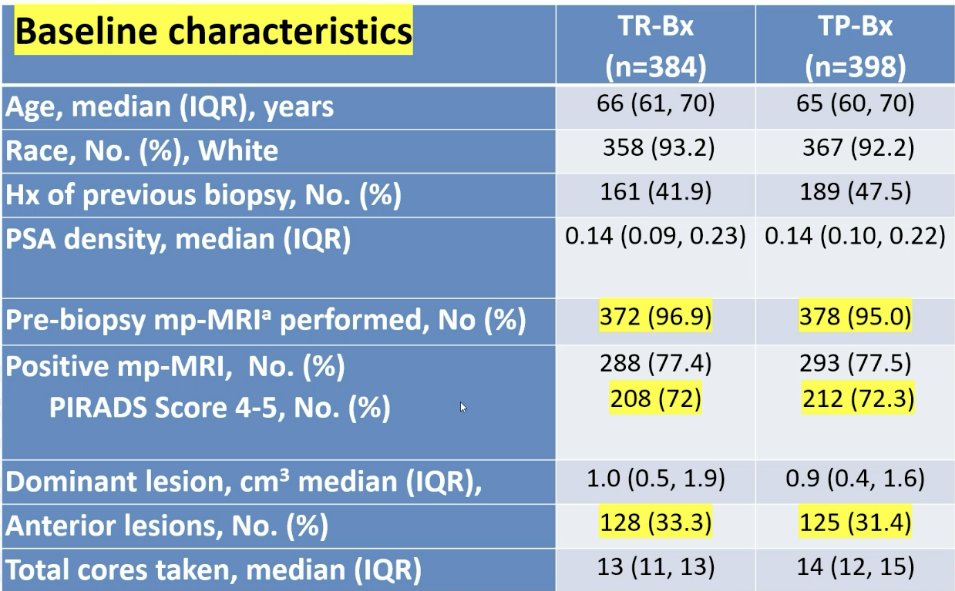
With regards to Grade Group ≥2 prostate cancer detection rates, transrectal biopsies detected such lesions in 47.1% of cases, compared to 43.2% for transperineal biopsy (OR: 0.72, 95% CI: 0.50 – 1.04). With regards to any grade detection, the detection rates were 72.1% and 70.4% for transrectal and transperineal biopsies, respectively.
Predictors of Grade Group ≥2 detection are summarized below. Notably, biopsy technique and presence of an anterior lesion were not predictive of this outcome.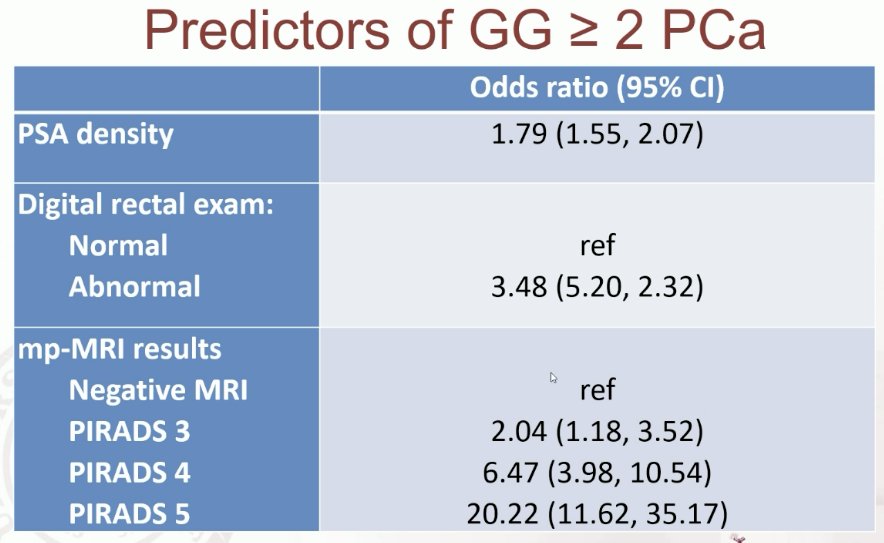
Subgroup analysis demonstrated that the Grade Group ≥2 detection was as follows:
- MRI targeted-biopsy (including prior negative biopsy):
- Transrectal: 56%
- Transperineal: 53%
- Anterior MRI lesion
- Transrectal: 42%
- Transperineal: 39%
- Negative MRI
- Transrectal: 16%
- Transperineal: 14%
- Biopsy-naïve men undergoing a targeted biopsy
- Transrectal: 59%
- Transperineal: 62%
- These results are consistent with those reported in other trials summarized below

An ongoing trial in this space is the UK-based TRANSLATE trial in biopsy-naïve men.
Limitations to the ProBE-PC trial include its performance at a single site using a fusion platform, with a limited number of operators and limited experience with transperineal biopsy, which may limit generalizability/external validity of these results. Conversely, potential strengths to this study include:
- Centralized procedure at a single site
- Fidelity to the study protocol with fewer violations
- Only 0.2% did not undergo the allocated procedure
- Compared to 6% in the PREVENT trial
- Software fusion for both transrectal and transperineal biopsies
- Other studies mixed fusion and visual estimation
- Pragmatic design representative of routine practice
- May improve generalizability
Presented by: Badar Mian, MD, FACS, Professor of Surgery, Department of Urology, Albany Med Health System, Albany, NY
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
References:

