(UroToday.com) The 2024 European Association of Urology (EAU) annual congress held in Paris, France was host to a joint session of the EAU and the Advanced Prostate Cancer Consensus (APCCC). Professor Karim Fizazi discussed the evidence for systemic therapy in patients with synchronous, low-volume metastatic hormone-sensitive prostate cancer (mHSPC).
Currently, the LATITUDE risk stratification and the CHAARTED volume schema are used to stratify mHSPC patients into low- and high-risk and volume groups, respectively:

What are some of the remaining questions for patients with low-volume mHSPC?
- What is the optimal duration for systemic treatments?
- Should we use bone protecting agents?
- Should we use 1, 2, or 3 systemic treatment options?
- Should their treatment differ compared to high-volume patients?
- Can/should we use biomarkers to guide therapy?
- Is there a role for local treatment of the primary site?
- Is there a role for directed treatment of the metastases?
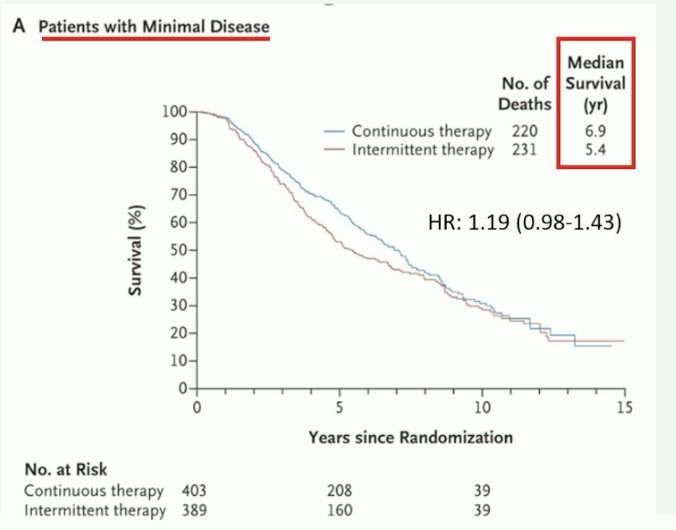
With regard to the optimal duration of systemic therapy, Professor Fizazi argued that continuous treatment remains the standard in mHSPC. While this trial did not evaluate systemic therapy intensifications with doublet or triplet therapy, the 2013 SWOG trial of continuous versus intermittent ADT for patients with mHSPC demonstrated that intermittent therapy was inferior to continuous ADT with regards to overall survival (although this remains inconclusive). This was most notable in the ‘minimal disease’ subgroup of patients with nodal only or bone-only disease limited to the pelvis and vertebral axis, whereby patients on continuous therapy had a median survival of 6.9 years, compared to 5.4 years for intermittent therapy. Additionally, he argued that the quality-of-life benefits remained modest with intermittent therapy.1 As such, he emphasized that given the current literature, continuous treatment remains the standard for mHSPC.
What about bone-protective agents? Data from two randomized trials has demonstrated that there is no role for monthly zoledronic acid (and likely denosumab) in patients with mHSPC.

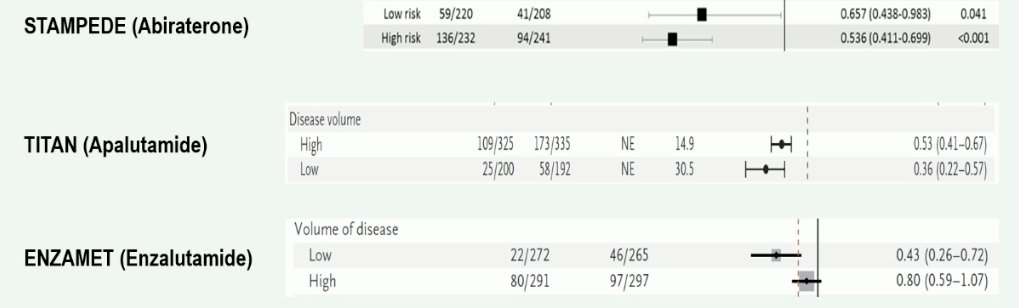
What about using the number of bone lesions to guide treatment decision-making? There are contrasting results from the CHAARTED and STAMPEDE trial of docetaxel for low-volume mHSPC. The CHAARTED trial demonstrated that there was no benefit for docetaxel in the low-volume subgroup, whereas the STAMPEDE trial suggests that there is a role for docetaxel in this cohort of patients.4,5
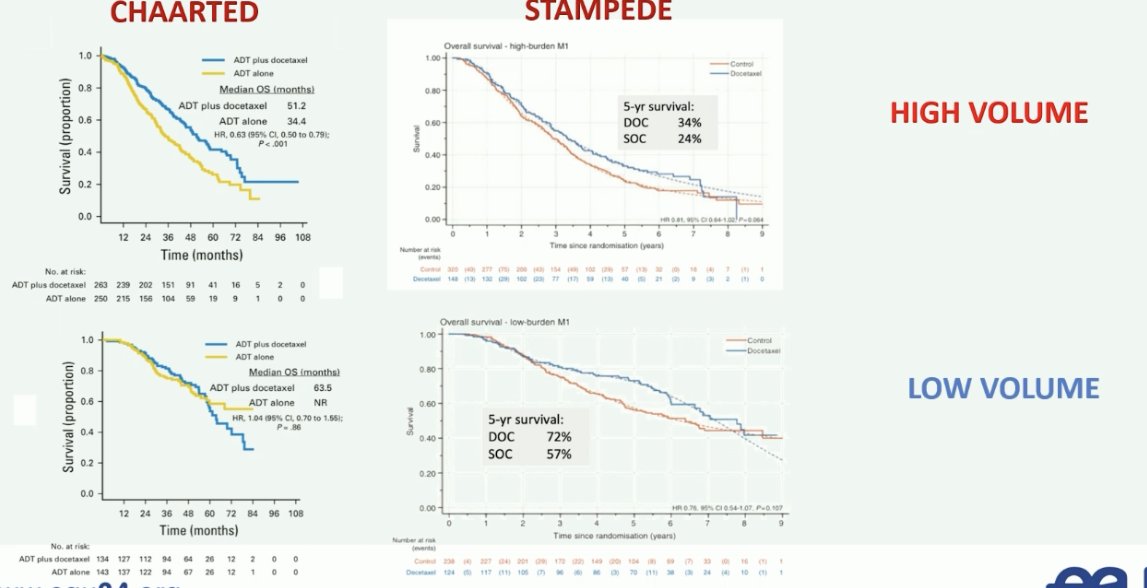
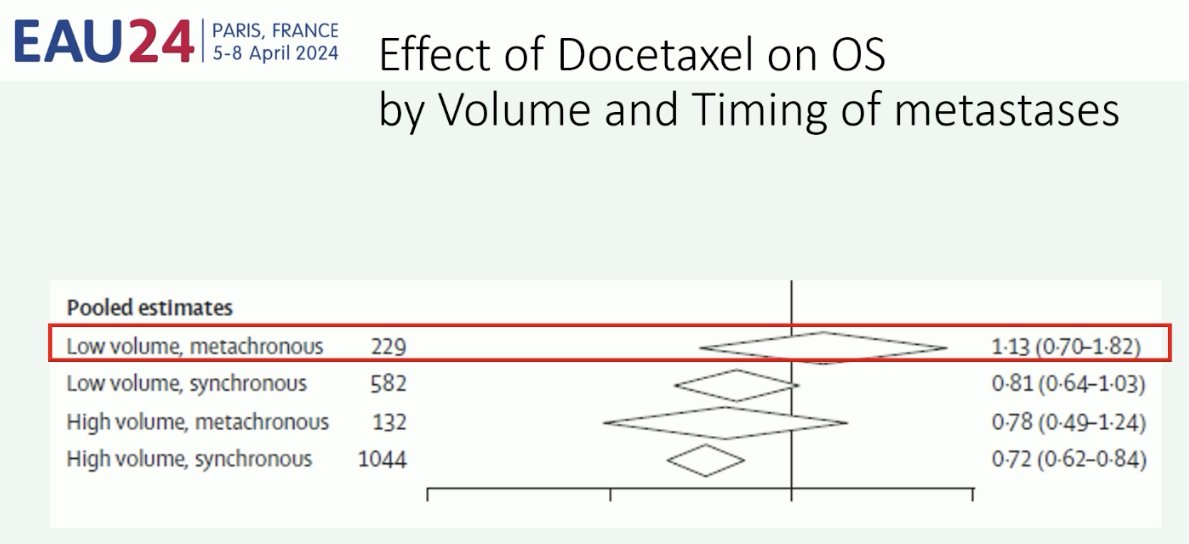
Professor Fizazi noted that one possible explanation for this discrepancy is that STAMPEDE mainly included patients with de novo disease, whereas CHAARTED included a significant number of patients with recurrent (i.e., metachronous) disease. This is of utmost importance given that patients with recurrent low volume disease appear to not derive a survival benefit from docetaxel, compared to patients with de novo (synchronous) low volume disease.6
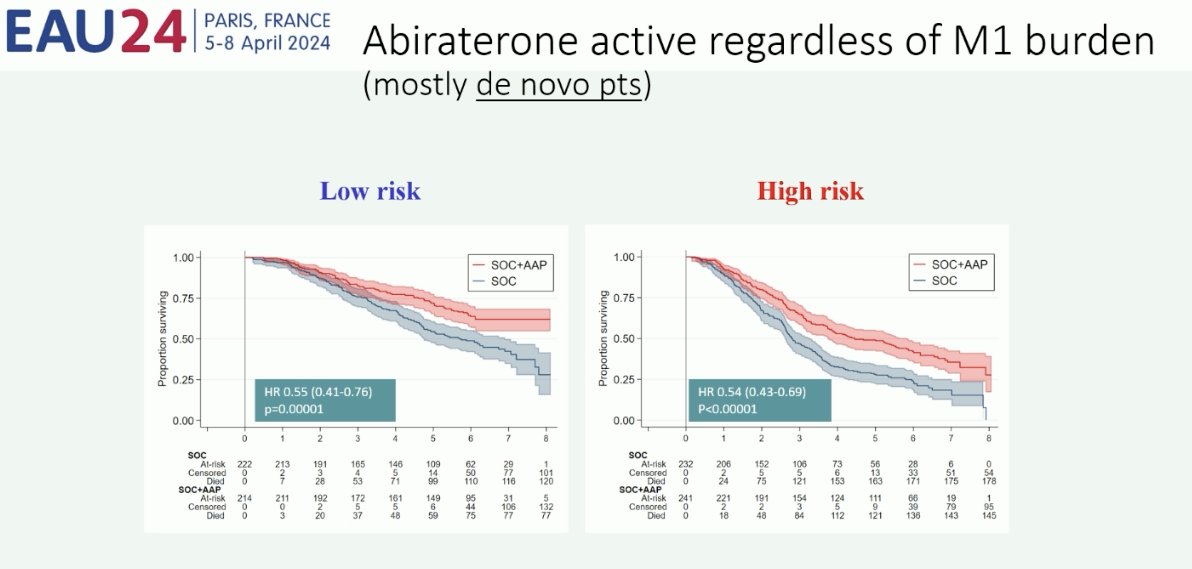
What about androgen receptor pathway inhibitors? In contrast to docetaxel, it appears that there is a consistent benefit for these agents in both low- and high-volume/risk groups, irrespective of the specific agent used. As such, Professor Fizazi argued that the number of metastatic lesions, and thus risk stratification, should be a secondary concern when considering androgen receptor pathway inhibitors for these patients.
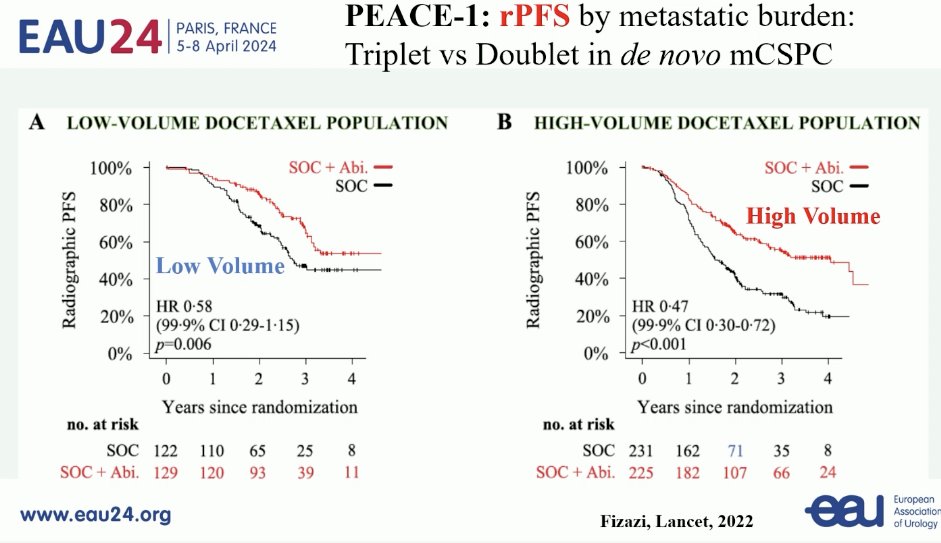
Do patients with synchronous, low-burden mHSPC benefit from triplet systemic therapy? Results of the PEACE-1 trial clearly demonstrate that there is a radiographic progression-free survival (rPFS) benefit to the addition of abiraterone to standard of care therapy (ADT +/- docetaxel) in patients with both de novo low- and high-volume disease.7 Whether this rPFS benefit translates to overall survival remains unknown, but Professor Fizazi noted that updated analysis of this outcome is underway and will be presented later in the year.
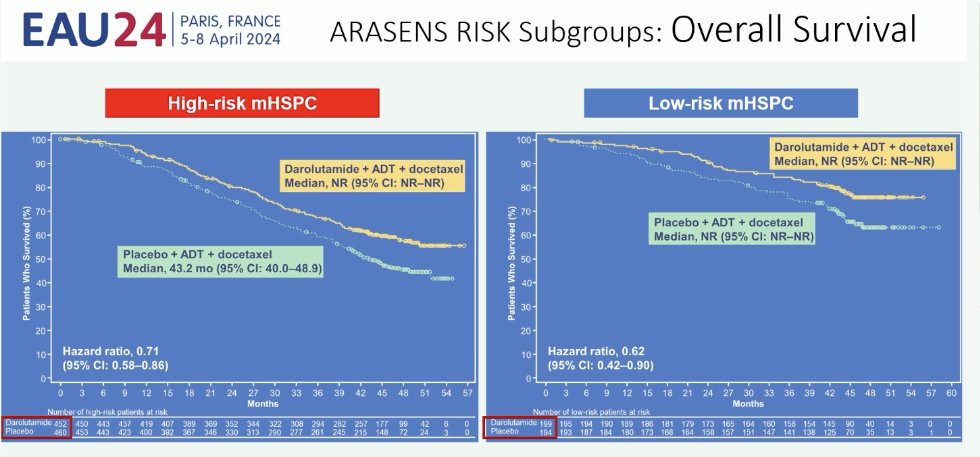
Subgroup analysis of ARASENS clearly demonstrates that there is a benefit to the addition of darolutamide to ADT + docetaxel, irrespective of risk/volume status.8
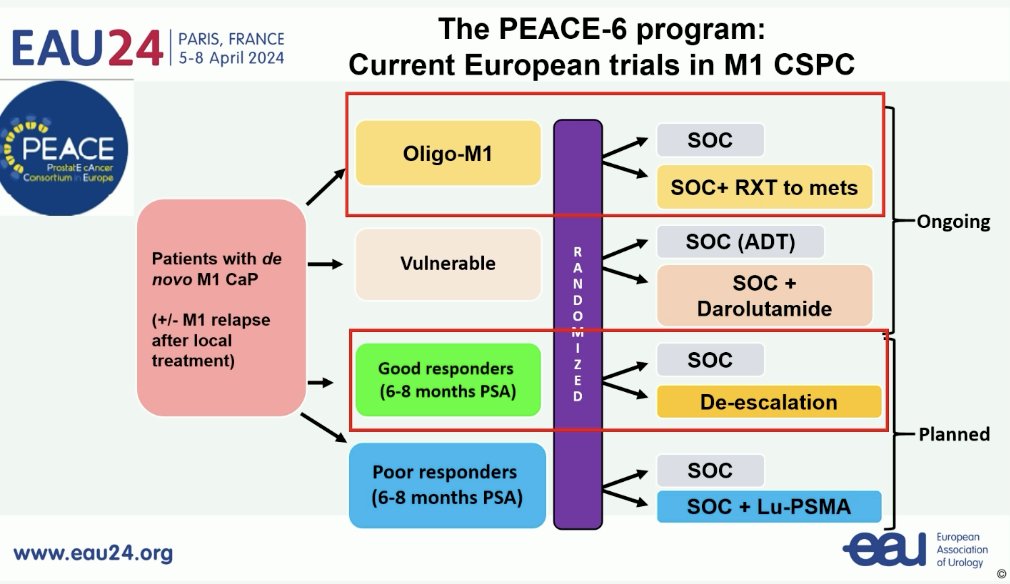
Ongoing trials in the mHSPC disease space include those evaluating the role of metastasis-directed therapy in patients with oligometastatic disease, treatment de-escalation in good responders, and treatment intensification with Lu-PSMA in poor responders.
Professor Fizazi concluded with the following take home messages for the treatment of patients with synchronous mHSPC:
- Low volume:
- Treat with ADT + an androgen receptor pathway inhibitor until disease progression
- ADT + docetaxel + an androgen receptor pathway inhibitor in fit, young patients with bone disease
- Prostate radiotherapy to prevent genitourinary symptoms (no overall survival benefit)
- High volume:
- Triplet therapy
- Consider prostate radiotherapy to prevent genitourinary symptoms
Presented by: Karim Fizazi, MD, PhD, Professor, Department of Medicine, Institut Gustave Roussy, Paris, France
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024References:
- Hussain M, Tangen CM, Berry DL, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368(13): 1314–25.
- Smith MR, Halabi S, Ryan CJ, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol. 2014;32(11): 1143–50.
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024): 1163-77.
- Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: Long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol 2018 Apr 10;36(11): 1080-7.
- Clarke NW, Ali A, Ingleby FC, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results in the STAMPEDE trial. Ann Oncol 2019 Dec 1;30(12):1992-2003.
- Vale CL, Fisher DJ, Godolphin PJ, et al. Which patients with metastatic hormone sensitive prostate cancer benefit from docetaxel: A systematic review and meta-analysis of individual participant data from randomized trials. Lancet Oncol. 2023;(7): 783-797.
- Fizazi K, Foulon S, Carles J, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomized, phase 3 study with a 2 x 2 factorial design. Lancet. 2022 Apr 30;399(10336):1695-1707.
- Hussain M, Tombal B, Saad F, et al. Darolutamide plus androgen-deprivation therapy and docetaxel in metastatic hormone-sensitive prostate cancer by disease volume and risk subgroups in the phase III ARASENS trial. J Clin Oncol. 2023 Jul 10;41(20):3595-3607.


