(UroToday.com) The 2024 European Association of Urology (EAU) annual congress held in Paris, France between April 5th and 8th was host to a joint session of the EAU and the Advanced Prostate Cancer Consensus (APCCC). Professor Elena Castro discussed whether germline genetic testing is necessary and/or helpful for the management of patients with synchronous metastatic hormone-sensitive prostate cancer (mHSPC).
Over the last decade, there have been significant advancements in defining the genomic landscape of prostate cancer.1-3 It is estimated that ~60% of the individual patient’s risk is attributable to genetic factors, with the prevalence of pathogenic germline variants progressively increasing with advancing stages of prostate cancer:
- Low-risk localized prostate cancer: 3% (similar to the general population)
- High-risk localized prostate cancer: 6%
- Metastatic prostate cancer: 8–12%
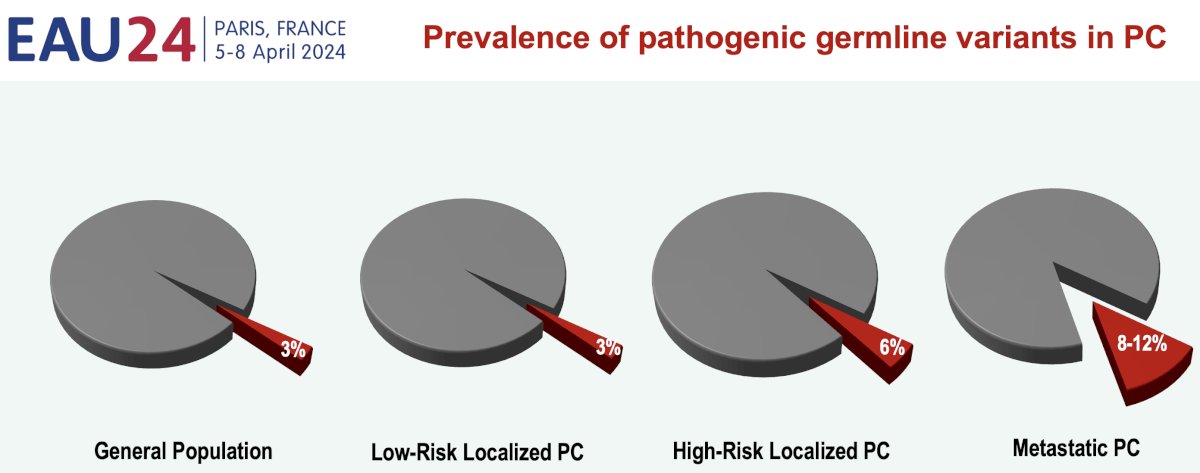
The exact prevalence of alterations differs from one study to another, depending on patient characteristics and genetic background; however, it has been consistently shown that BRCA2 mutations are the most prevalent mutations.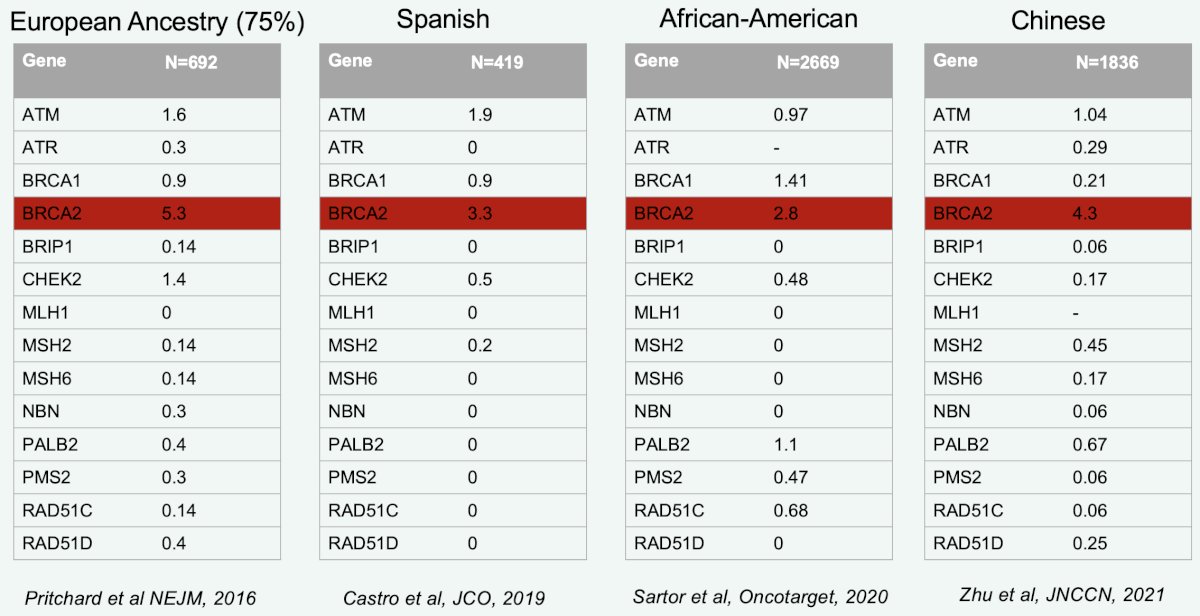
Detailed guidance on the performance of germline genetic testing for prostate cancer patients comes from the 2019 Philadelphia Prostate Cancer Consensus Conference. Among patients with metastatic castrate sensitive or resistant prostate cancer, their recommendation is to use a broad panel that includes, at least, BRCA1, BRCA2, and mismatch mutation repair (MMR) genes. Testing for ATM may be considered, and additional genes may be tested for based on the personal and family history. Importantly, we should always check if any mutation identified on tumor profiling could have a germline origin.
Among patients with non-metastatic prostate cancer, testing should be considered for those with an Ashkenazi Jewish ancestry, advanced disease, Grade Group ≥4, and intraductal/cribriform pathology.
Among unaffected men with a family history of prostate cancer, the recommendations for who should be tested and for which specific genes is summarized below.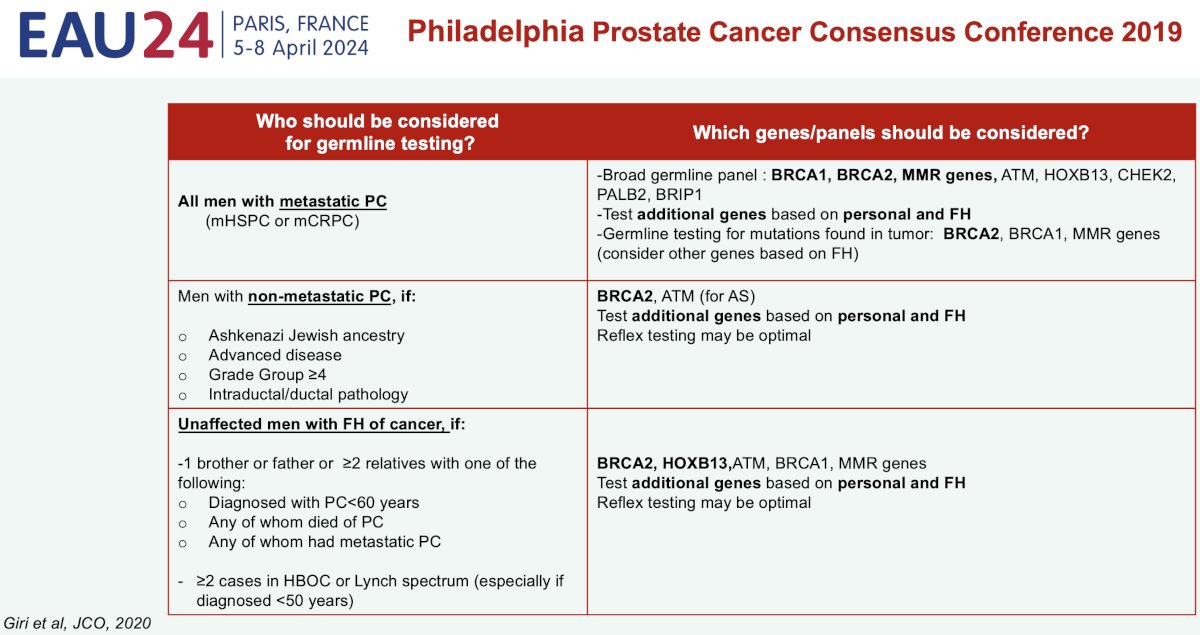
Reasons to perform germline testing in prostate cancer include:
- Establishing prognosis
- Deciding treatment
- Assessing familial cancer risk
Germline BRCA2 mutations have been demonstrated to be prognostic in prostate cancer. Among patients with localized disease undergoing active surveillance, the rate of upgrading is significantly higher for patients with such mutations.5 Metastatic rates are also worse for patients with localized disease undergoing a radical prostatectomy or radiotherapy.6 This trend continues into the metastatic castrate-resistant setting, whereby it has been established that those with BRCA2 mutations have worse survival outcomes.2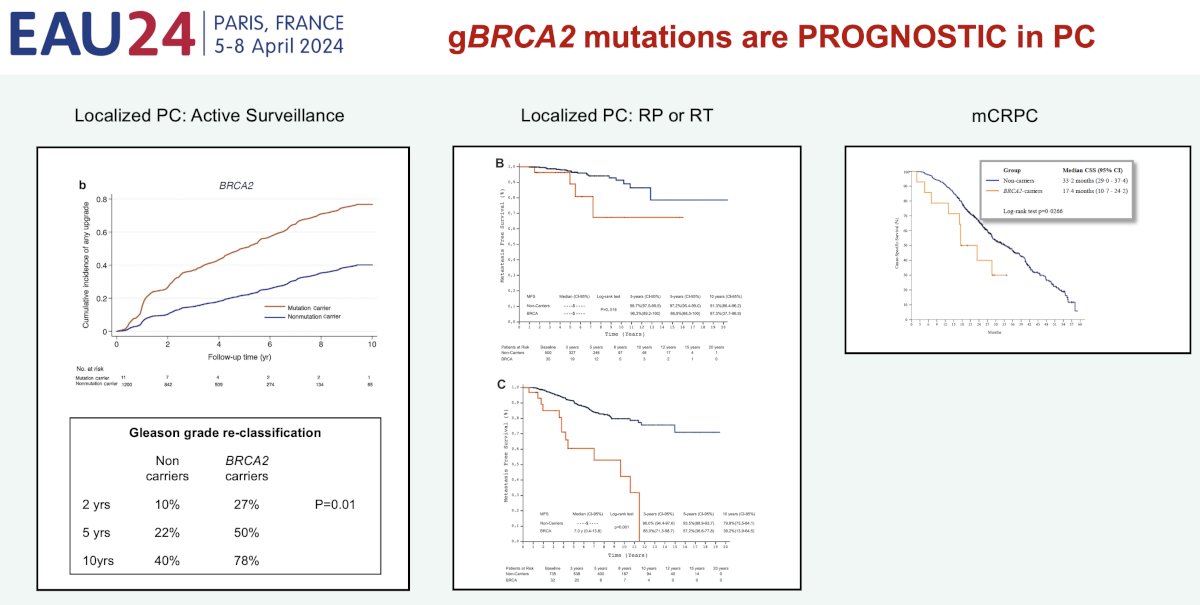
Secondly, such mutations also act as predictive biomarkers, whereby patients with germline (and somatic) BRCA1/2 mutations experience superior responses to PARP inhibitor therapy.7,8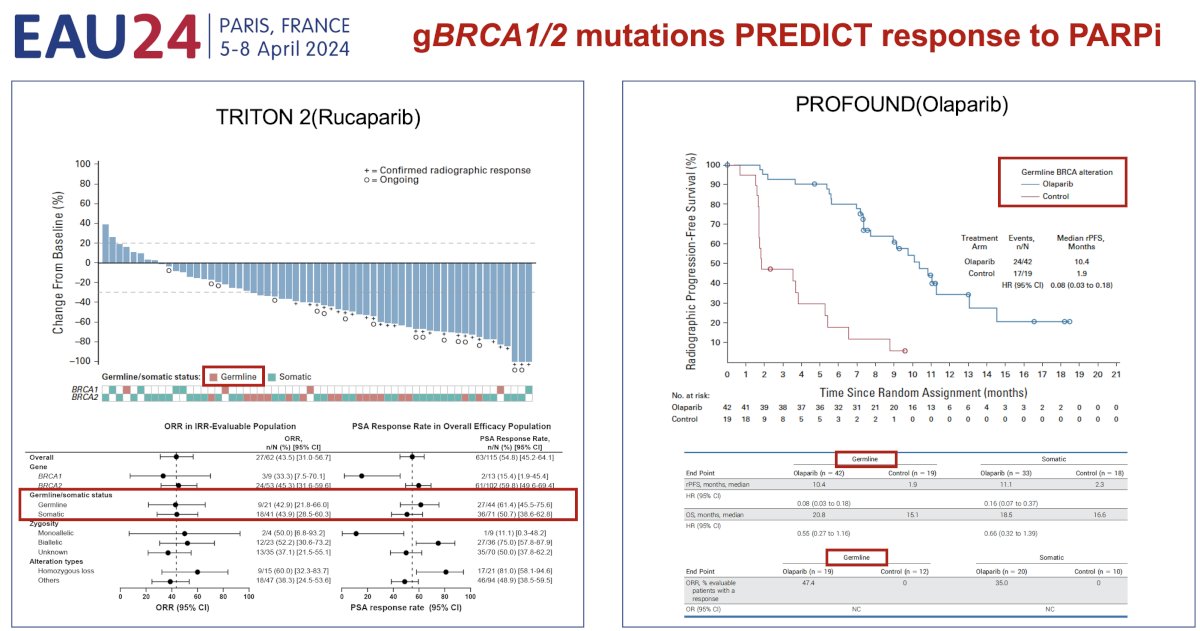
While identification of alterations through somatic tumor testing is sufficient for guiding treatment, there is some evidence that patients with germline mutations derive a superior survival benefit with PARP inhibitors, compared to those with somatic mutations.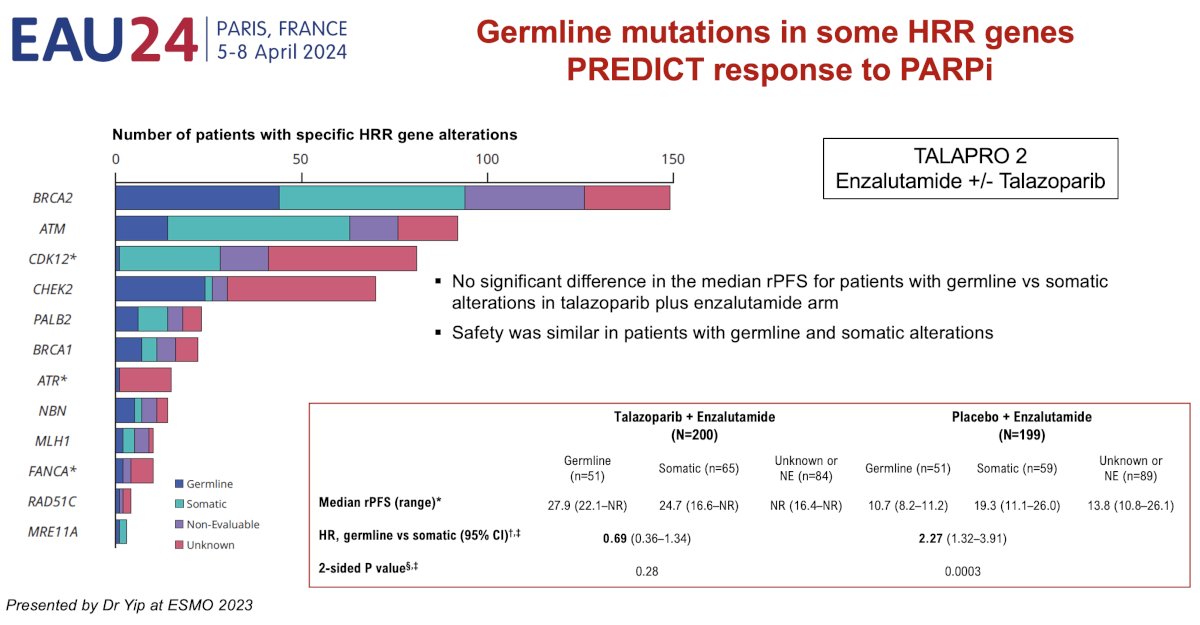
Thirdly, germline testing can help identify DNA damage response (DDR) mutations that may result in cancer predisposition syndromes. Depending on the specific alteration, carriers may be at increased risk of prostate cancer or other malignancies, such as pancreatic, gastric, or colon cancer. Family members, including daughters, may be at increased risk of breast and ovarian cancer, underlining the implications of such mutations. Early identification of such mutation carriers would prompt cascade testing, allowing for the early identification of relatives at risk who could benefit from prevention and early detection programs.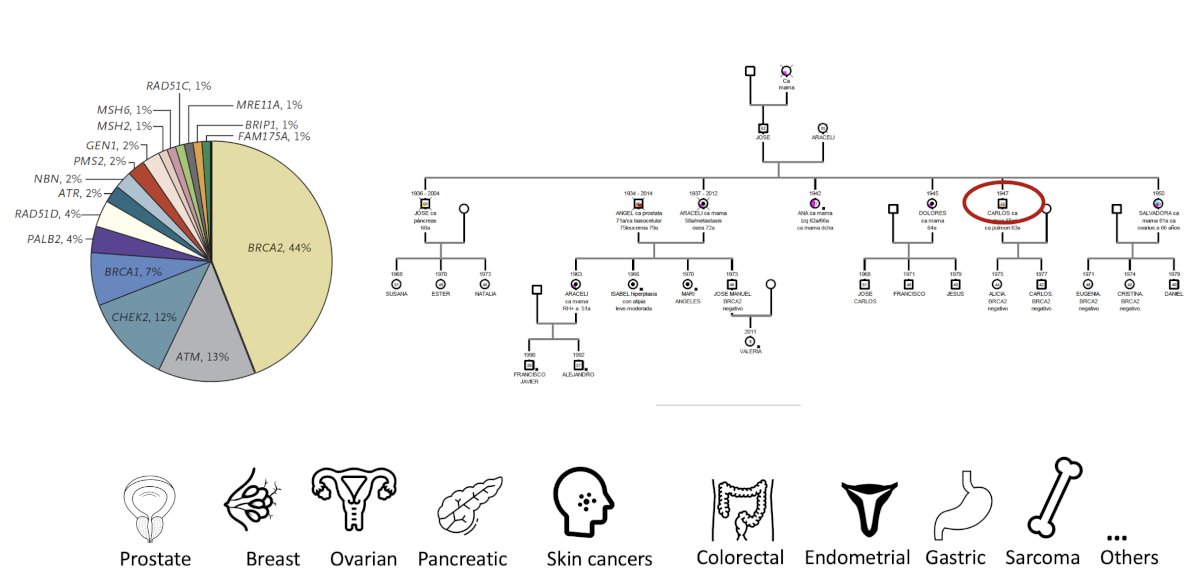
Given the approval of targeted therapies, particularly when considering the relatively high prevalence of germline mutations in prostate cancer, including numerous actionable ones, the updated guidelines of most scientific societies currently recommend tumor and germline testing for high-risk localized and metastatic prostate cancer.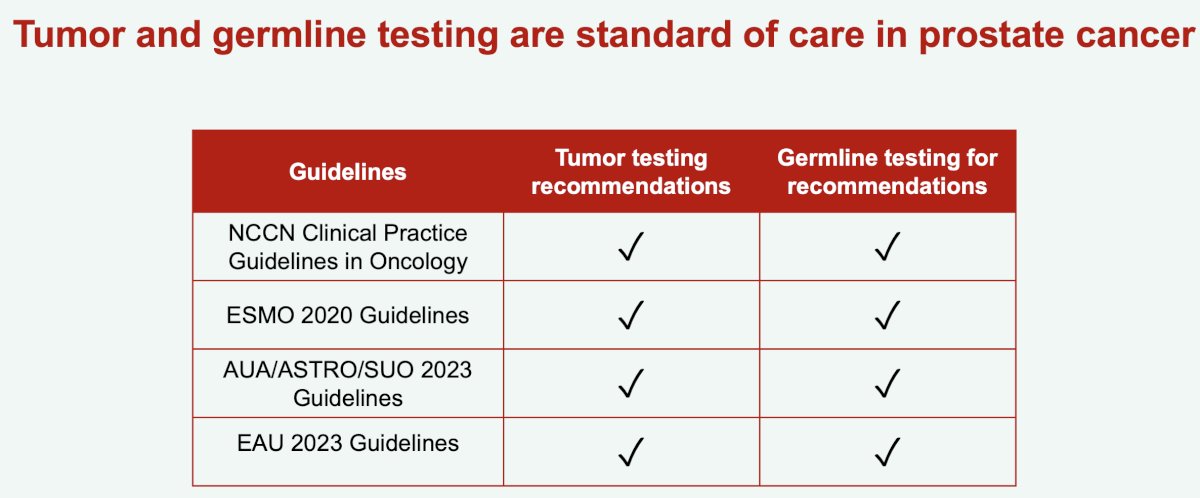
It is critical that both germline and somatic testing be performed, given that germline-only testing misses ~50% of patients potentially eligible for PARP inhibitor therapy.9
Conversely, tumor-only testing misses ~7% of germline pathogenic variants in prostate cancer.10 The tumor sampling quality and somatic copy number alterations may mask germline variants. This is demonstrated by tumor-only sequencing detecting:
- 100% of nonsense and missense single nucleotide variations and insertions/deletions
- 55% of deletions/duplications
- 0% of variants in high homology regions, intronic single nucleotide variants
As such, Dr. Castro argued that we should offer germline testing to patients at high risk of being carriers, despite negative tumor-sequencing results.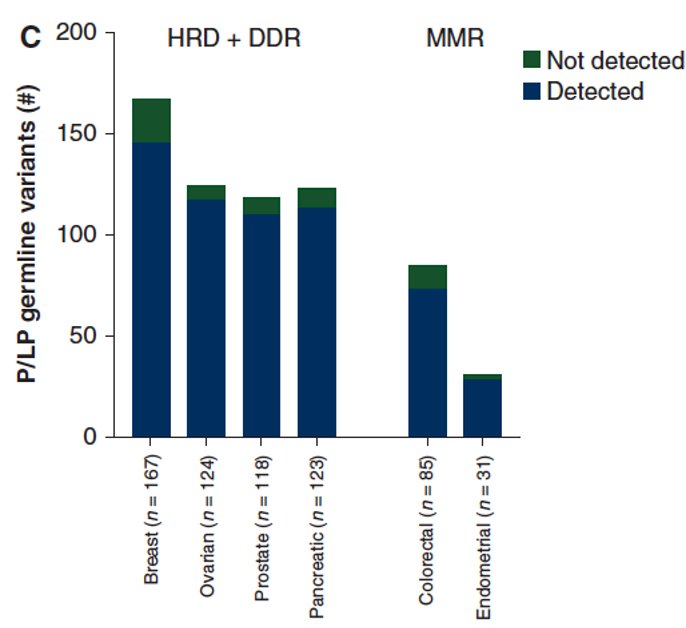
It appears that ~70% of mutations of a germline origin are related to prostate cancer when somatic tumor testing is performed. Conversely, 12% of detected germline mutations are unrelated to prostate cancer.11
Dr. Castro concluded her presentation as follows:
- Germline testing is important for the optimizing the care of prostate cancer patients, but also for the prevention and early detection of tumors amongst relatives
- A broad panel should be performed: BRCA1, BRCA2, MMR genes, ATM, HOXB13, CHEK2, PALB2. Other genes should be tested for based on personal and family history.
- -Germline testing should be offered to:
- All prostate cancer patients with metastatic disease
- For patients with non-metastatic disease
- Family history of prostate or cancers genetically related to prostate cancer
- Personal history of a cancer predisposition syndrome
- Ashkenazi Jewish ancestry
- High-risk or very high-risk disease
- Ductal / intraductal histology
- If tumor testing is performed, germline testing should also be offered if patients have:
- A negative tumor-sequencing results but have a personal or family history that may suggest a cancer predisposition syndromes
- Tumor findings with a potential germline origin
- Tumor gene panel did not include some genes associated with cancer predisposition syndromes
Presented by: Professor Elena Castro, MD, MS, PhD, Medical Oncology, Hospital Universitario, Madrid, Spain
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
References:- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N Engl J Med. 2016;375(5): 443-453.
- Castro E, Romero-Laorden N, Del Pozo A, et al. PROREPAIR-B: A Prospective Cohort Study of the Impact of Germline DNA Repair Mutations on the Outcomes of Patients With Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. 2019;37(6): 490-503.
- Nicolosi P, Ledet E, Yang S, et al. Prevalence of Germline Variants in Prostate Cancer and Implications for Current Genetic Testing Guidelines. JAMA Oncol. 2019;5(4): 523-528.
- Giri VN, Knudsen KE, Kelly WK, et al. Implementation of Germline Testing for Prostate Cancer: Philadelphia Prostate Cancer Consensus Conference 2019. J Clin Oncol. 2020;38(24): 2798-2811.
- Carter HB, Helfand B, Mamawala M, et al. Germline Mutations in ATM and BRCA1/2 Are Associated with Grade Reclassification in Men on Active Surveillance for Prostate Cancer. Eur Urol. 2019;75(5): 743-749.
- Castro E, Goh C, Leongamornlert D, et al. Effect of BRCA Mutations on Metastatic Relapse and Cause-specific Survival After Radical Treatment for Localised Prostate Cancer. Eur Urol. 2015;68(2): 186-193.
- Abida W, Campbell D, Patnaik A, et al. Rucaparib for the Treatment of Metastatic Castration-resistant Prostate Cancer Associated with a DNA Damage Repair Gene Alteration: Final Results from the Phase 2 TRITON2 Study. Eur Urol 2023;84: 321-30.
- Hussain M, Mateo J, Fizazi K, et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;383:2345-57.
- Olmos D, Lorente D, Alameda D, et al. Treatment patterns and outcomes in metastatic castration-resistant prostate cancer patients with and without somatic or germline alterations in homologous recombination repair genes. Ann Oncol. 2024;S0923-7534(24)00043-7.
- Terraf P, Pareja F, Brown DN, et al. Comprehensive assessment of germline pathogenic variant detection in tumor-only sequencing. Ann Oncol. 2022;33(4): 426-433.
- Kuzbari Z, Bandlamudi C, Loveday C, et al. Germline-focused analysis of tumour-detected variants in 49,264 cancer patients: ESMO Precision Medicine Working Group recommendations. Ann Oncol. 2023;34(3): 215-227.


