(UroToday.com) The 2024 European Association of Urology (EAU) annual meeting featured a plenary session on personalized approaches in high-risk and metastatic prostate cancer, and a state of the art lecture by Dr. Karim Fizazi discussing how to personalize treatment in metastatic hormone sensitive prostate cancer (mHSPC). Dr. Fizazi started by emphasizing that yes, of course, the number of bone metastases has prognostic value. But, does counting bone metastases help with treatment decision making? Before 2023, common thoughts were that for low volume mHSPC there would be no use of docetaxel, utilization of ARPIs, and radiotherapy to the primary. For high volume mHSPC, patients would receive triplet systemic therapy (ADT + docetaxel + ARPI), with no radiotherapy to the primary tumor. Based on volume data from CHAARTED1 and STAMPEDE,2 there was clinical benefit to using docetaxel in high volume disease, however, in low volume disease there was a benefit in the STAMPEDE population but not in the CHAARTED cohort:
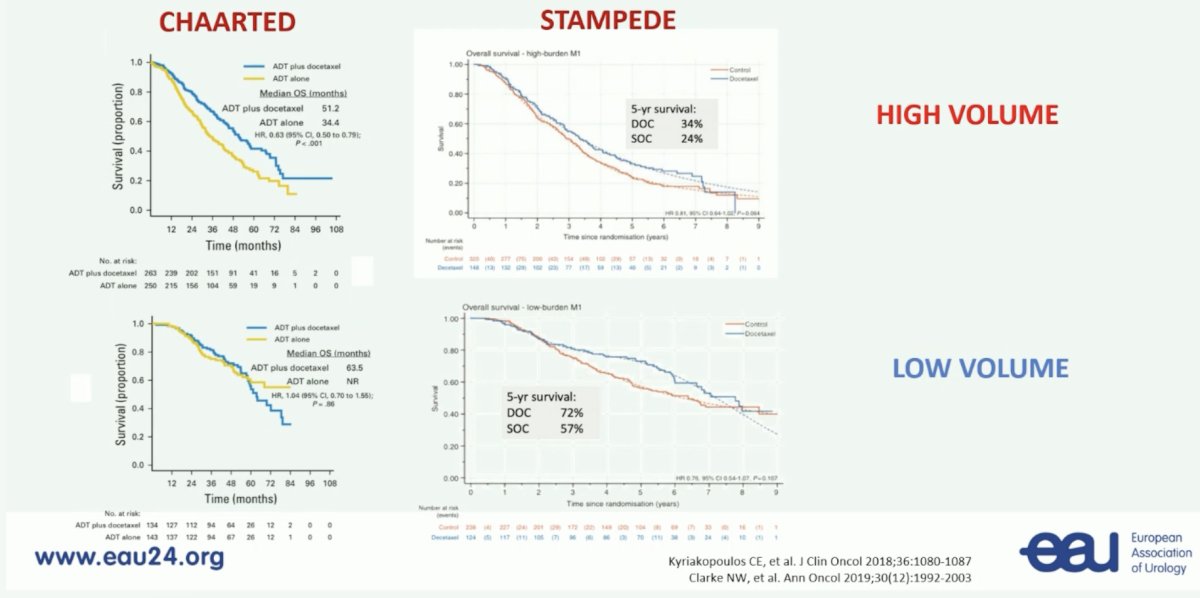
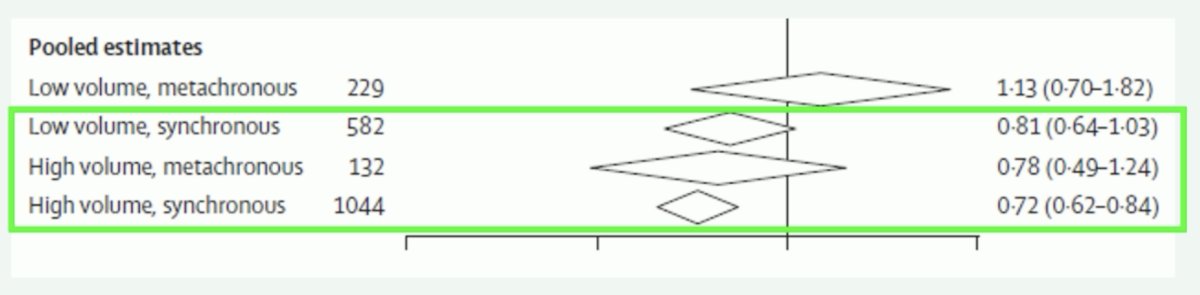
Dr. Fizazi then discussed the importance of timing and burden of metastases in M1 HSPC, specifically whether this is de novo low versus high burden of disease, or recurrent low versus high burden of disease. One specific outlier with improved survival are those patients with M1 relapsed disease and oligometastatic:

Based on work from Vale and colleagues,3 low volume, metachronous patients do not have a docetaxel benefit for improved survival (HR 1.13, 95% CI 0.70-1.82). This is contrary to the other three disease states that see a survival benefit with docetaxel:
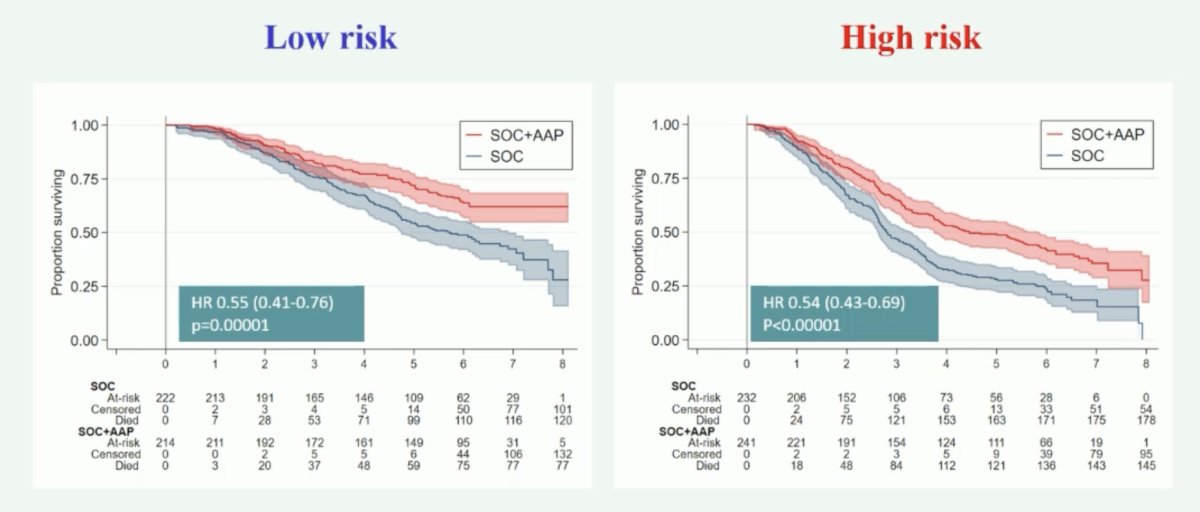
Generally, second generation ARPIs are active regardless of M1 burden (amongst mostly de novo patients). The following shows the benefit in low and high-risk patients with the addition of abiraterone + standard of care:
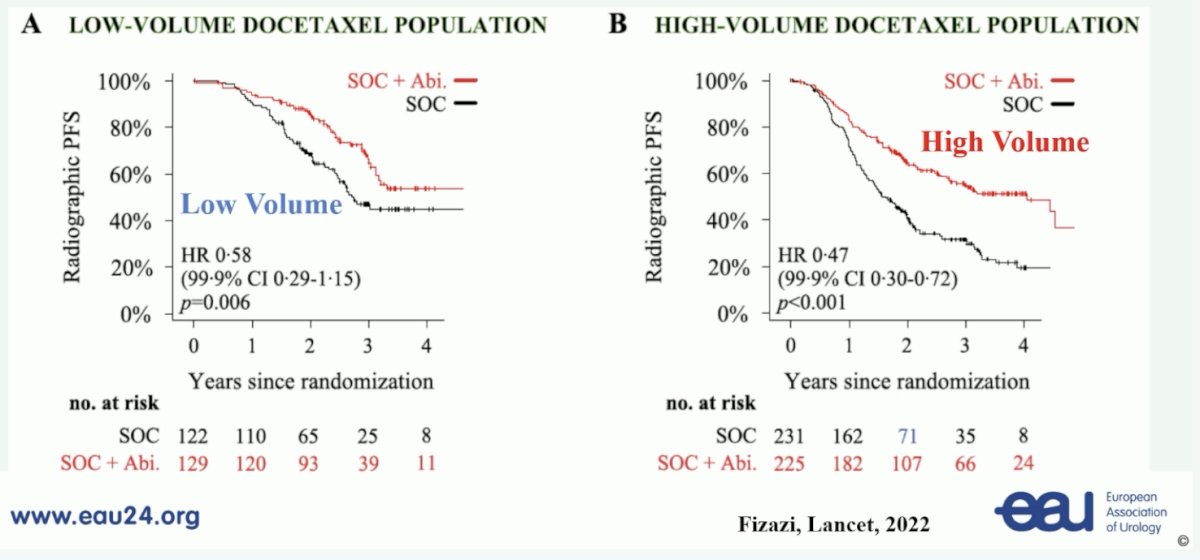
With regards to triplet therapy, the PEACE-1 trial4 showed a benefit in radiographic progression free survival for the triplet vs double therapy in both the low volume (HR 0.58, 99.9% CI 0.29-1.15) and high volume (HR 0.47, 99.9% CI 0.30-0.72) setting:
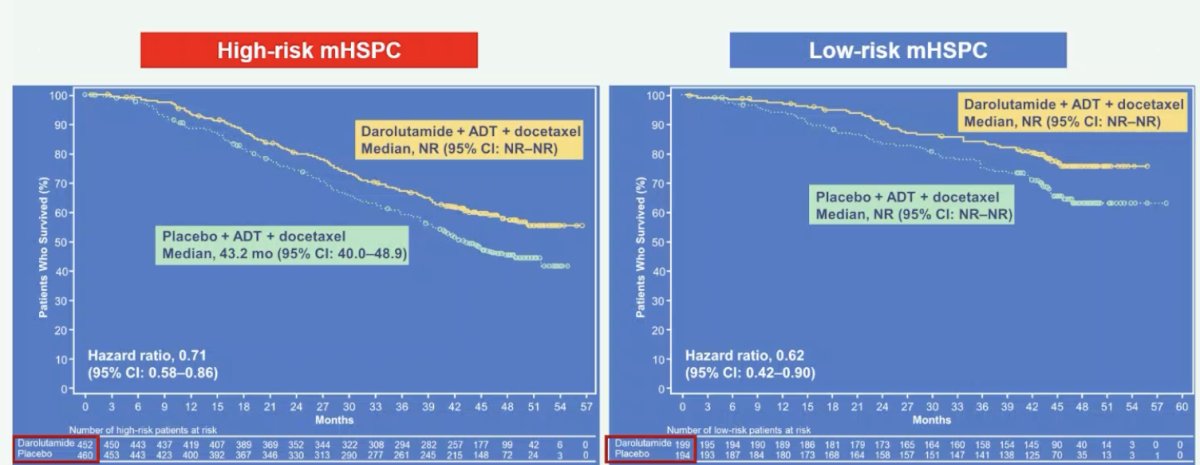
When thinking about mHSPC risk groups, the ARASENS trial showed that both high (HR 0.71, 95% CI 0.58-0.86) and low risk (HR 0.62, 95% CI 0.42-0.90) mHSPC showed a significant benefit to the addition of darolutamide to ADT + docetaxel versus ADT + docetaxel alone5:
Regarding expected data in 2024 and beyond, Dr. Fizazi highlighted the potential impact of personalizing the use of metabolic modulators, such as metformin mHSPC (STAMPEDE) or statins and aspirin in mCRPC (PEACE-4). Another aspect is personalizing treatment based on biomarkers. This includes:
- PARP inhibitors in BRCA patients (TALAPRO-3): completed
- Targeting PSMA with LuPSMA (PSMAddition): completed
- Akt inhibitors in PTEN loss (CAPItello-281): completed
Next, Dr. Fizazi emphasized the importance of an 8-month PSA response and the associated prognostic impact in mHSPC. Data from PEACE-1 showed that those with an 8 month PSA < 0.2 ng/mL had improved overall survival for both ADT +/- docetaxel and ADT +/- docetaxel + ADT:

The PEACE-6 program highlights the current European trials in M1 HSPC, particular importance is the de-escalation of therapy in those that are good responders with an undetectable PSA at 6-8 months:

Dr. Fizazi concluded his presentation by discussing how to personalize treatment in mHSPC in 2024 with the following conclusions:
- For low volume, de novo mHSPC:
- ADT + ARPI
- ADT + docetaxel + ARPI for those that are young, fit, and have bone predominant disease
- Prostate radiotherapy
- For high volume, de novo mHSPC:
- Triplet systemic therapy
- Consider prostate RT to prevent GU symptoms
- For low volume, relapsed mHSPC:
- ARPI likely needed (scarce data)
- SBRT to metastatic sites is currently being tested
Hopefully the aforementioned will continue to improve/change with biomarkers.
Presented by: Karim Fizazi, MD, PhD, Gustave Roussy Cancer, Université Paris-Saclay, Villejuif, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
References:
- Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: Long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol 2018 Apr 10;36(11):1080-1087.
- Clarke NW, Ali A, Ingleby FC, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results in the STAMPEDE trial. Ann Oncol 2019 Dec 1;30(12):1992-2003
- Vale CL, Fisher DJ, Godolphin PJ, et al. Which patients with metastatic hormone sensitive prostate cancer benefit from docetaxel: A systematic review and meta-analysis of individual participant data from randomized trials. Lancet Oncol. 2023 Jul(7):783-797.
- Fizazi K, Foulon S, Carles J, Roubaud G, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomized, phase 3 study with a 2 x 2 factorial design. Lancet. 2022 Apr 30;399(10336):1695-1707.
- Hussain M, Tombal B, Saad F, et al. Darolutamide plus androgen-deprivation therapy and docetaxel in metastatic hormone-sensitive prostate cancer by disease volume and risk subgroups in the phase III ARASENS trial. J Clin Oncol. 2023 Jul 10;41(20):3595-3607.


