At this point the patient was offered 3 treatment options:
1. Radical cystectomy
2. Bladder preservation with chemoradiation
3. Active surveillance
Dr. Solsona presented the option of active surveillance for patients with clinical stage T2-T4a and complete response after chemotherapy (clinical stage T0). A study using the National Cancer Database (NCDB) analyzing 19,404 unselected patients with clinical stage T2-T4a N0M0 disease who underwent either radical cystectomy or maximal TURBT + chemotherapy demonstrated that the overall survival was slightly better in the radical cystectomy group (36.2% vs. 32.9%, p<0.0001, with a HR of 1.02 (95% CI 1.01-1.3)1. In a meta-analysis including 18 studies and 518 patients comparing TURBT with chemotherapy to radical cystectomy, it was shown that the 5-year overall survival was 61% in the TURBT+ chemotherapy arm with cancer specific survival of 72% and 61.5% patients being cystectomy free2. Data from other centers demonstrated similar favorable outcomes for this specific treatment strategy. Additionally, in a SWOG (S0219) phase 2 trial including 74 patients with clinical stage T2-T4a disease, all patients were given neoadjuvant chemotherapy for 3 weeks. Out of the 34 patients reaching clinical stage T0 disease, 10 underwent radical cystectomy and 24 patients remained on observation. The 2-year overall survival rate was similar between the groups (70% in the radical cystectomy group compared to 76% in the observation group)3.
There is also data comparing TURBT + chemotherapy to trimodal therapy in patients reaching clinical stage T0 disease after chemotherapy + TURBT or after trimodal therapy. These data showed at least comparable results between both modalities, as can be seen in table 1, with a disease specific survival ranging between 80.8%-93% in the TURBT+ Chemotherapy group compared to 76.6%-85% in the trimodal therapy group.
Table 1 – Comparison of studies analyzing trimodal therapy and TURBT + Chemotherapy:
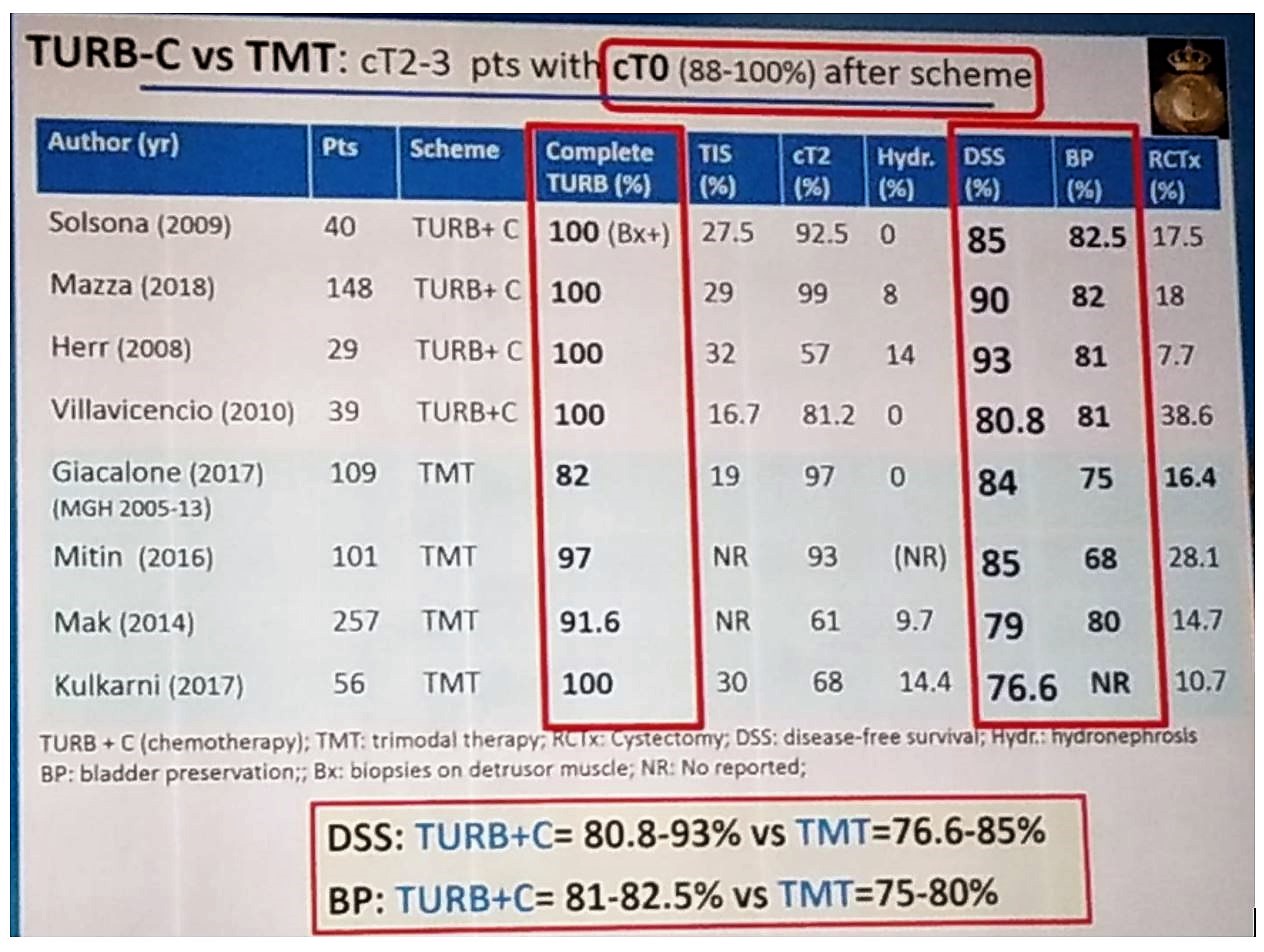
Dr. Solsona concluded that this data is robust enough to show that active surveillance is the best approach for patients with clinical stage T2-T4a that have received chemotherapy and have reached a clinical stage T0 disease.
References:
1. Audenet F et al. Effectiveness of Transurethral Resection plus Systemic Chemotherapy as Definitive Treatment for Muscle Invasive Bladder Cancer in Population Level Data. J Urol. 2018 Nov;200(5):996-1004. doi: 10.1016/j.juro.2018.06.001. Epub 2018 Jun 4
2. Moran GW, Li G, Robins DJ, Matulay JT, McKiernan JM, Anderson CB. Systematic Review and Meta-Analysis on the Efficacy of Chemotherapy with Transurethral Resection of Bladder Tumors as Definitive Therapy for Muscle Invasive Bladder Cancer. Bladder Cancer. 2017;3(4):245-258. Published 2017 Oct 27. doi:10.3233/BLC-170134
3. deVere White RW, Katz MH, Steinberg GD. The case for neoadjuvant chemotherapy and cystectomy for muscle invasive bladder cancer. J Urol. 2009 May;181(5):1994-7. doi: 10.1016/j.juro.2009.02.052. Epub 2009 Mar 14.
Presented by: Eduardo Solsona, MD, PhD, Department of Urology, Instituto Valenciano de Oncologia, Valencia, Spain
Written By: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 34th European Association of Urology (EAU 2019) #EAU19, conference in Barcelona, Spain from March 15-19, 2019.


