At this point the patient was offered 3 treatment options:
- Radical cystectomy
- Bladder preservation with chemoradiation (Trimodal therapy)
- Active surveillance
The first point was that there is a paradigm shift in contemporary oncology: organ conservation is commonplace in many other cancers – laryngeal, anal, breast, esophageal, and limb sarcomas. Also, it is important to remember that radical cystectomy is a life-altering procedure with many associated physiologic, biologic, cognitive and emotional changes.
Second, the morbidity of radical cystectomy is quite significant. Data have shown that at centers of excellence, 64% of patients undergoing radical cystectomy have more than 1 complication, 13% have grade 3-5 complications, 26% are readmitted, and 2.7% of patients die within 90 days of the procedure.1,2 No differences in these numbers have been shown when open radical cystectomy was compared to robotic radical cystectomy. Moreover, it is important to remember that the recurrence rates in the pelvis are not low following radical cystectomy, approaching 40%, and late complications are considerable as well. Lastly, the development of renal insufficiency with cystectomy is quite substantial as well, with >70% of patients demonstrating renal deterioration ten years after surgery. Over 50%of the patients have new onset stage 3 chronic kidney disease, and 3.5% of patients will progress to hemodialysis. 3,4,5
Third, radical cystectomy is not being performed in 50% of patients – which is a huge unmet need, that trimodal therapy can fill (Figure 1). 6
Figure 1 – Percentage of patients undergoing radical cystectomy:

Fourth, trimodal therapy is still very much a surgical approach, and urologists are still the ones leading this treatment strategy, as can be seen in figure 2. The TURBT and salvage cystectomy are key to the success of trimodal therapy.7
Fifth, long-term results of trimodal therapy are excellent and comparable to radical cystectomy.7 Furthermore, the results are even better in trimodal therapy in patients with a complete response. 7 Data have shown that patients treated with trimodal therapy have an 84% 9-year survival rate with less than 15% of patients requiring salvage radical cystectomy.7 The best data comes from the Toronto (Princess Margaret Cancer Center) experience, comparing patients who underwent radical cystectomy to those treated with trimodal therapy. The results demonstrate a 5-year disease-specific survival rate of 73.2% and 76.6% for radical cystectomy and trimodal therapy patients, respectively, p=0.49 (Figure 3). 8
Figure 2 – The role of the urologist in trimodal therapy
 Figure 3 – Trimodal treatment compared to radical cystectomy – the Toronto experience:
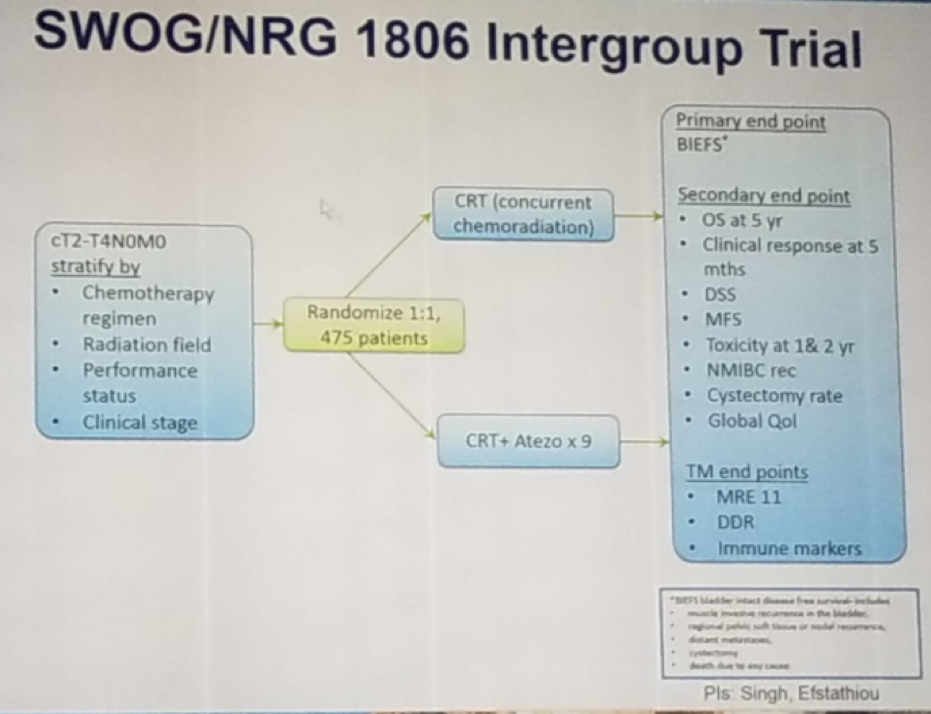
Figure 3 – Trimodal treatment compared to radical cystectomy – the Toronto experience: Sixth, Concurrent chemotherapy is important to the success of trimodal therapy. There are plenty of radio-sensitizing drugs that can be used including cisplatin, paclitaxel, 5-FU, Mitomycin C, Gemcitabine, and carbogen/nicotinamide. There are also many options for patients who cannot receive cisplatin. Currently, there is an ongoing randomized trial comparing standard trimodal therapy to standard trimodal therapy + atezolizumab (Figure 4), which we eagerly await its results.
Sixth, Concurrent chemotherapy is important to the success of trimodal therapy. There are plenty of radio-sensitizing drugs that can be used including cisplatin, paclitaxel, 5-FU, Mitomycin C, Gemcitabine, and carbogen/nicotinamide. There are also many options for patients who cannot receive cisplatin. Currently, there is an ongoing randomized trial comparing standard trimodal therapy to standard trimodal therapy + atezolizumab (Figure 4), which we eagerly await its results.Figure 4 – Ongoing randomized trial comparing standard trimodal therapy to the combination of trimodal therapy with Atezolizumab:
Seventh, long term toxicity is acceptable and quality of life after bladder preservation is good and even better than after radical cystectomy. Data have shown that trimodal therapy is associated with better sexual quality of life, better-informed decision making, fewer concerns about appearance, less life interference from cancer or cancer treatment, modestly higher bowel function, similar urinary scores, and modestly higher general quality of life.9,10
Eighth, Superficial recurrences can be managed conservatively in patients treated with trimodal therapy. Approximately 25% of patients developed non-muscle invasive tumors after complete response to trimodal therapy. However, 60% of them were recurrence-free after TURBT and BCG. Overall similar tolerability, toxicity, and outcomes were seen when compared to non-radiated patients.
Ninth, trimodal therapy is supported by numerous guidelines., including the National Comprehensive Cancer Network (NCCN), the AUA and the EAU guidelines.
The last point discussed the fact that we are approaching an era of biomarker-driven management of muscle-invasive bladder cancer. This novel tool may potentially help us decide which patients should be referred for radical cystectomy and which should be treated with trimodal therapy (Figure 5).11
Figure 5 – The potential role of biomarkers in deciding which patients to treat with Trimodal therapy:
 Dr. Efstathiou summarized his great talk, stating that in clinically matched patients, survival is comparable in the modern area between radical cystectomy and trimodal therapy. Over 85% of contemporary patients treated with trimodal therapy get to keep their own bladders with good long term quality of life. Trimodal therapy is not a non-surgical treatment: maximal TURBT and salvage radical cystectomy are important components of this strategy, and the urologist is a key member of the multidisciplinary team. Trimodal therapy is supported by multiple guidelines and it is important we advocate for multidisciplinary engagement.
Dr. Efstathiou summarized his great talk, stating that in clinically matched patients, survival is comparable in the modern area between radical cystectomy and trimodal therapy. Over 85% of contemporary patients treated with trimodal therapy get to keep their own bladders with good long term quality of life. Trimodal therapy is not a non-surgical treatment: maximal TURBT and salvage radical cystectomy are important components of this strategy, and the urologist is a key member of the multidisciplinary team. Trimodal therapy is supported by multiple guidelines and it is important we advocate for multidisciplinary engagement.Presented by:
Maurizio Brausi, Director Urology Ausl Modena, Chairman ESOU, Azienda Unità Sanitaria Locale Modena, Modena, Italy
Jason Efstathiou, MD, radiation oncologist Massachusetts General Hospital, Boston, Massachusetts
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 34th European Association of Urology (EAU 2019) #EAU19 conference in Barcelona, Spain, March 15-19, 2019.
References:
1. Donat et al. Eur Urol 2009
2. Bochner et al. Eur Urol 2015
3. Rouanne et al. Clinical Genitourinary Cancer 2015
4. Eisenberg et al. J. Urol 2014
5. Zabell et al. J Urol 2015
6. Gray et al. Eur Urol 2012
7. Giacalone et al. Eur Urol 2017
8. Kulkarni G. et al. JCO 2017
9. Efstathiou et al. JCO 2009
10. James et al. NEJM 2012
11. Miyamoto et al. Lancet Oncol 2018
Further Related Content: What Can We Expect from Imaging - Case-Based Debate No Evidence of Disease After Neoadjuvant Chemotherapy for MIBC: What Next?


