However, the latter recommendation is based on results obtained in the pre-MRI era. Longitudinal active surveillance studies from Toronto have demonstrated that the long-term metastatic rate is low (2.8%) and that those developing metastasis likely had Gleason >6 disease 2. The authors hypothesized that mpMRI characteristics might assist physicians in the identification of Gleason 3+4 patients suitable for active surveillance regardless of the number of positive scores.
For this study, there were 309 patients with Gleason 3+4 prostate cancer at biopsy who underwent MRI-targeted biopsy plus systematic cores and radical prostatectomy between 2016 and 2018. Prostate biopsy and prostatectomy specimens were evaluated by dedicated uropathologists. Misclassification was defined as non-organ confined or ISUP grade group ≥3 disease at radical prostatectomy. Multivariable logistic regression analyses assessed the association between mpMRI parameters (extracapsular extension [ECE] and maximum lesion diameter) and misclassification after adjusting for confounding variables. The diameter of the lesion at mpMRI was dichotomized according to the most informative cut-off predicting misclassification. A novel risk classification was subsequently proposed based on clinical characteristics and mpMRI findings. A comparison of the proportion of patients eligible for active surveillance and the rates of misclassification was performed between men selected for active surveillance according to the new imaging criteria vs. established criteria of low volume Gleason 3+4 (≤2 positive cores).
The median patient age was 65 years (IQR 60-69), median PSA at surgery was 7 (IQR 5-9) ng/mL, mean prostate volume was 40 (IQR 33-54) mL, and median lesion diameter was 10 (IQR 9-15) mm. Overall, there were 39 (13.2%) patients that had suspicious ECE at mpMRI, and 185 (59.9%) patients experienced misclassification. On multivariable analyses, ECE at mpMRI (OR 3.2, 95%CI 1.12-8.9) and lesion diameter (OR 1.08, 95%CI 1.01-1.15) were associated with the risk of misclassification; the ROC-derived area under the curve of the model was 75%. The most informative cut-off of lesion diameter for misclassification was 12 mm. Patients without ECE at mpMRI and with a lesion <12 mm were classified as low risk regardless of the number of positive cores. The proportion of patients potentially eligible for active surveillance increased from 18.1 to 22.5% when adopting the new mpMRI criteria compared to the inclusion of only men with 1-2 positive cores.
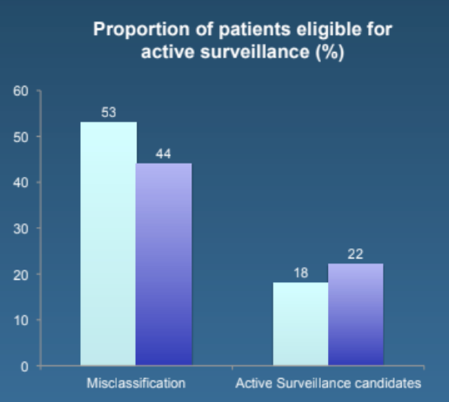
The rate of misclassification was lower in intermediate-risk patients selected according to the new mpMRI criteria vs. those selected according to the number of positive cores (44.6 vs. 53.6%).
This is an important study as it continues to provide further granularity as to who among Gleason 3+4 prostate cancer patients may be safely eligible for active surveillance.
Dr. Gandaglia concluded this study with the following take-home messages:
- Information obtained at mpMRI can assist physicians in the identification of men with Gleason 3+4 more likely to experience misclassification
- Men with biopsy Gleason 3+4 without ECE and with a lesion <12 mm at mpMRI biopsy could be included in AS protocols regardless of the number of positive cores at biopsy thus expanding AS indications while decreasing the rates of misclassification at the same time.
Presented by: Giorgio Gandaglia, IRCCS Ospedale San Raffaele, Dept. of Urology, Milan, Italy
Written by: Zachary Klaassen, MD, MSc, Assistant Professor of Urology, Georgia Cancer Center, Augusta University - Medical College of Georgia, Twitter: @zklaassen_md at the 34th European Association of Urology (EAU 2019) #EAU19 conference in Barcelona, Spain, March 15-19, 2019.
References:
1. Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2017;71(4):618-629.
2. Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015;33(3):272-277.


