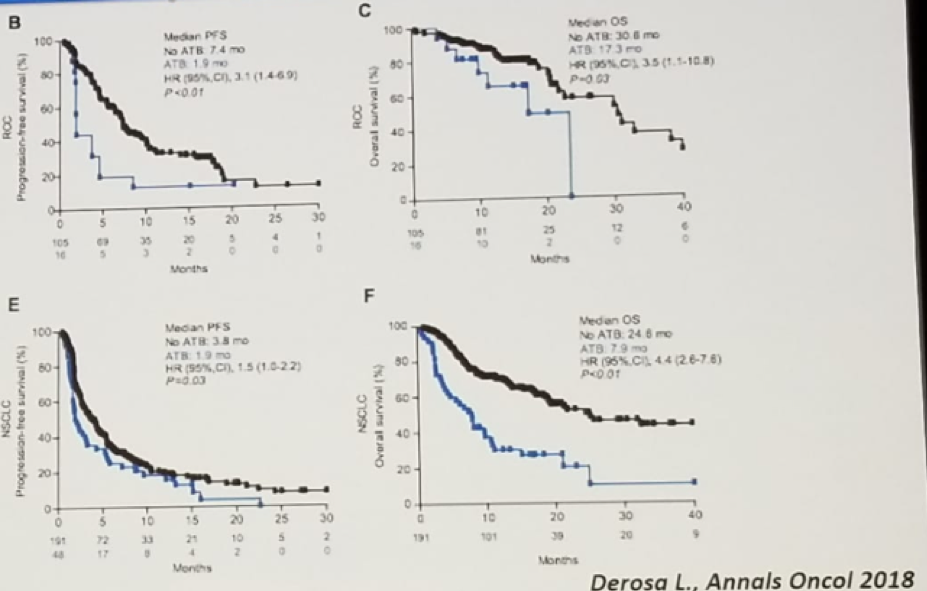
The antitumor efficacy of anticancer immunotherapy requires gut microbiota. There is also data in mouse models showing that antibiotics use is associated with worse outcomes under immune checkpoint inhibitors. These studies demonstrated that antibiotic exposure remained independently associated with worse progression-free survival and overall survival, as shown in figure 1.
Figure 1 – Antibiotic exposure is associated with worse progression-free survival and overall survival:
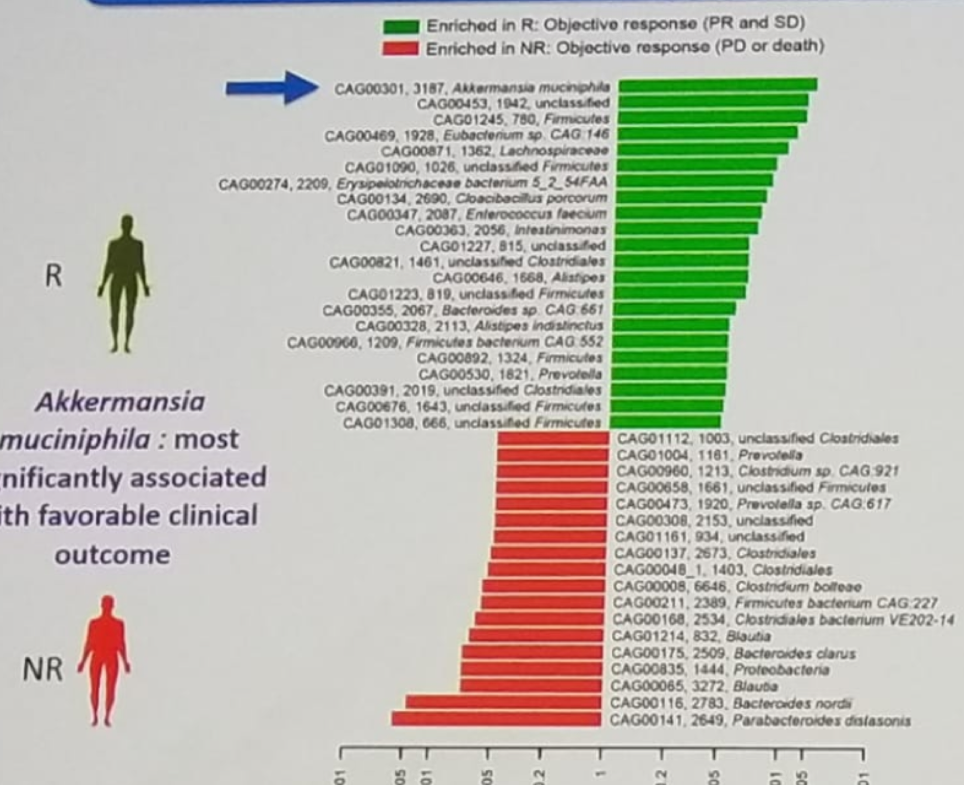
The working hypothesis is that antibiotic exposure can change the composition of the gut microbiome (at least in a transient way). Dr. Albiges speculates that dysbiosis (gut microbiome alteration) and ultimately the composition of the microbiome might affect the therapeutic efficacy of immunotherapy. This leads to the important hypothesis that gut microbiome might be able to predict the efficacy of PD-1/PD-L1 blockade. There is data showing that response to PD-1 inhibitor is associated with distinct gut microbiota species, as shown in figure 2. The microbiota species of Akkermansia muciniphila has been shown to be most associated with favorable clinical outcome. But there are other microbiota species that have shown an increased response to PD-1 inhibitors.
Figure 2 – Response to PD-1 inhibitors is associated with distinct gut microbiota:
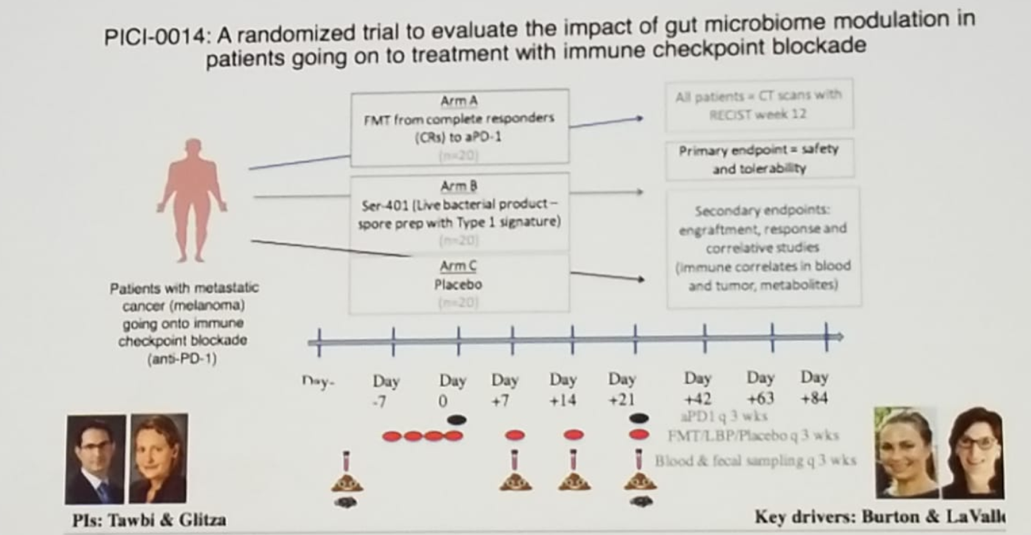
In an experiment in a mouse model conducted by Dr. Albiges’s team, sensitivity to PD-1 was restored following the intervention of feces transplantation, and therefore, at least in mice, modulation of the microbiota can enhance response to immunotherapy. Fecal microbiota transplantation, which is a safe and effective approved therapy for C. Difficile infection, is currently being used experimentally in cancer. There is a planned randomized trial (PICI-0014) to evaluate the impact of gut microbiome modulation in patients undergoing treatment with immune checkpoint inhibitors. The trial design is shown in figure 3.
Figure 3 – A proposed randomized trial evaluating the impact of gut microbiome modulation in patients undergoing treatment with immune checkpoint inhibitors:
There is a microbial population that specifically resides in the genitourinary tract. They may influence the development of genitourinary malignancies. However, the microbiome outside the genitourinary tract may also influence genitourinary cancers and treatment response. This leads to the question of whether we can perhaps design a preventative trial in the near future based on this hypothesis.
Dr. Albiges concluded her talk, emphasizing that microbiota affects immunity and therapeutic response in cancer mouse models, suggesting that modulation of the microbiome can enhance response to immune checkpoint inhibitors and prevent toxicity. There is currently an ongoing paradigm shift in oncology from tumor cells to the immune system to the host and its environment, and much more will be revealed on this topic in the near future.
Presented by: Laurence Albiges, MD, Ph.D., Head of Genitourinary Tumor Unit, Department of Cancer University of Paris Sud - Paris, France
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 34th European Association of Urology (EAU 2019) #EAU19, conference in Barcelona, Spain from March 15-19, 2019.


