The PREVAIL study design is shown in figure 1. The PREVAIL study was halted after a preplanned interim analysis, due to the superiority of enzalutamide compared with placebo.
Figure 1 – PREVAIL study design:
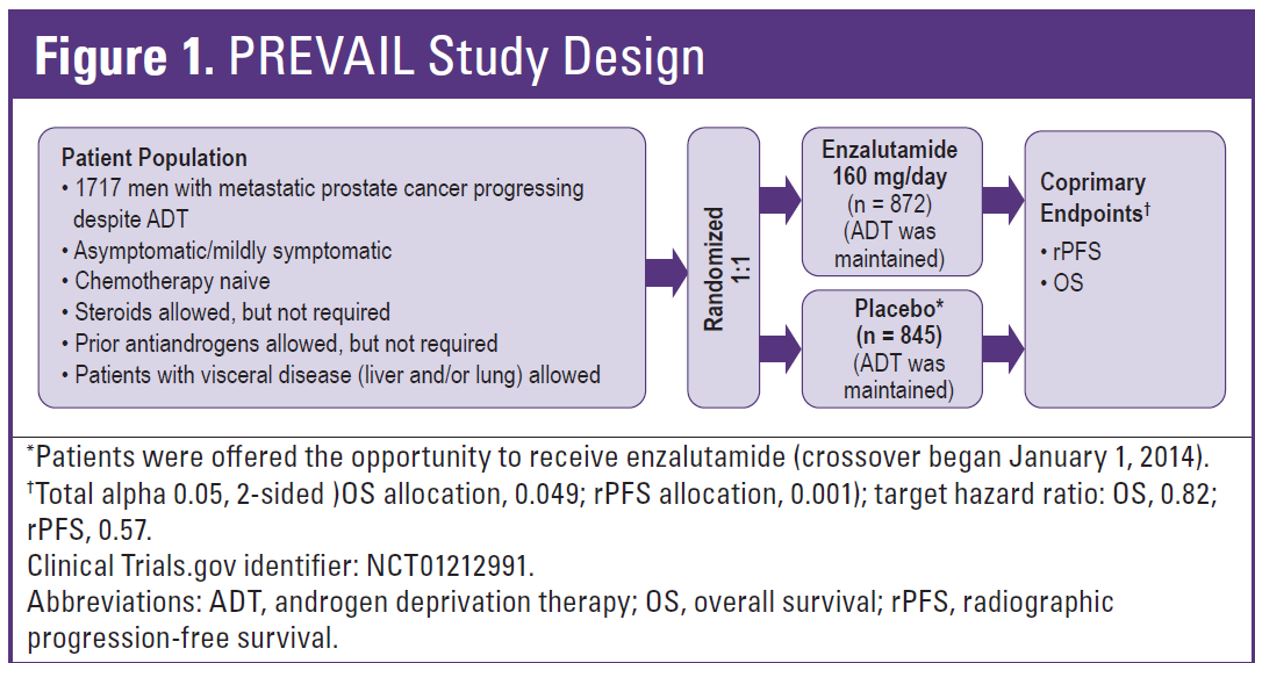
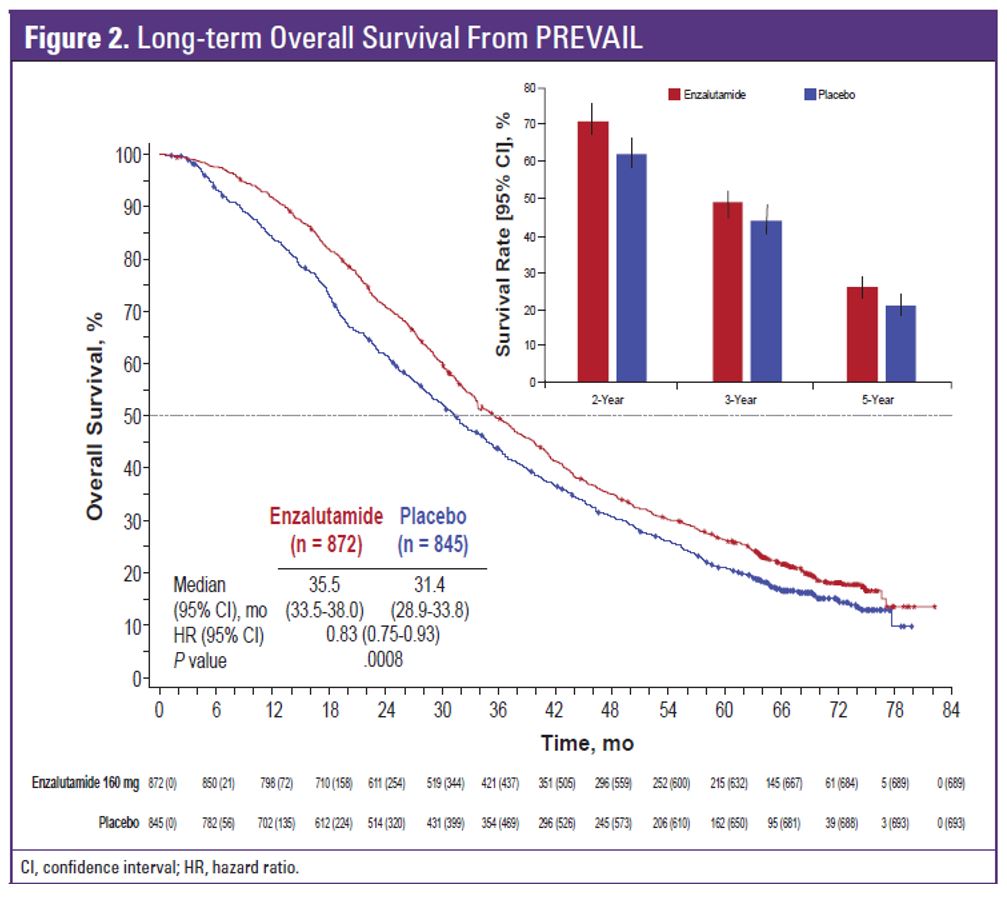
A total of 1717 men were randomized in PREVAIL between 2010 and 2012. Table 1 demonstrates the patient flow in the study. At the extended overall analysis data cutoff (September 2017), 955 patients from the original enzalutamide arm and 435 from the placebo arm had entered the open-label extension or were in long term follow-up. Enzalutamide was shown to reduce the risk of death by 29% (HR 0.71, 95% 0.6-0.84, p<0.001). The median overall survival was 32.4 months in the enzalutamide arm vs. 30.2 months in the placebo arm (figure 2). At the five-year overall survival analysis, enzalutamide reduced the risk of death by 17% (HR 0.83, 95% CI 0.75-0.93, p=0.008). At a median follow-up of 69 months, the median overall survival was 35.5 months in the enzalutamide arm vs. 31.4 months in the placebo arm.
Table 1:
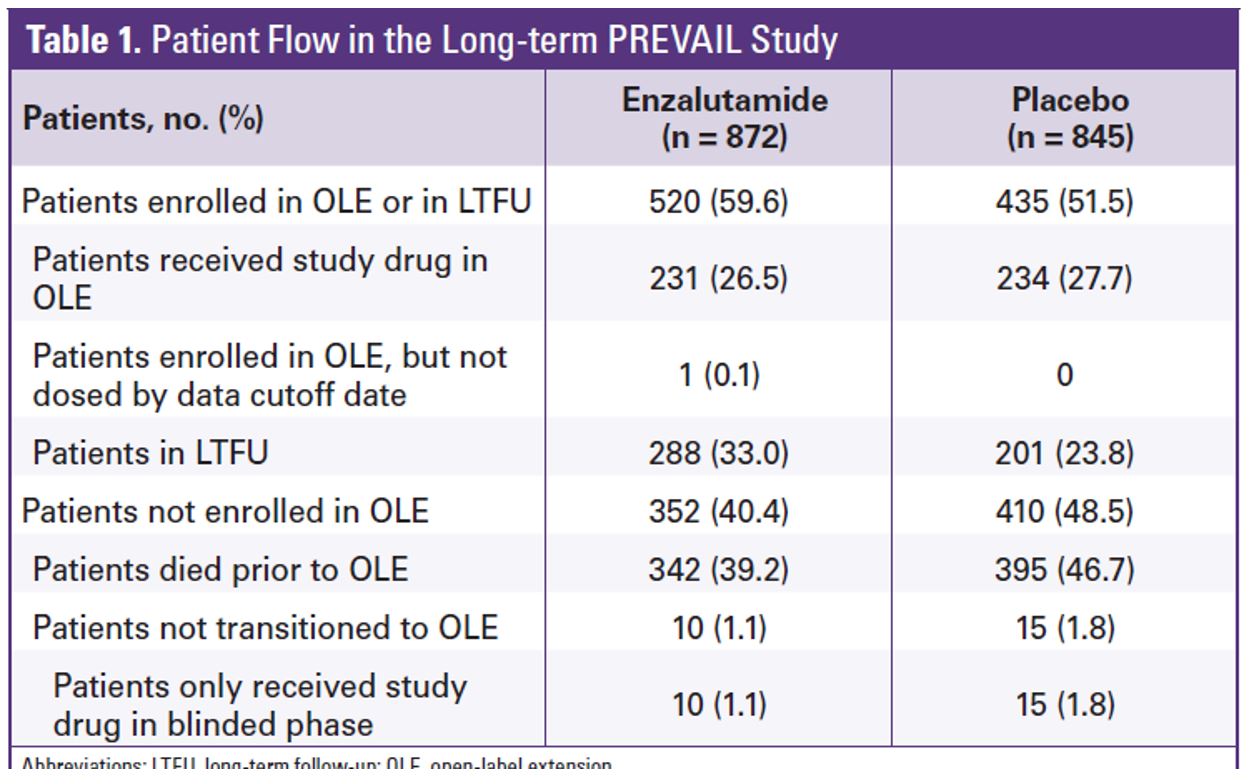
Figure 2: Long term overall survival:
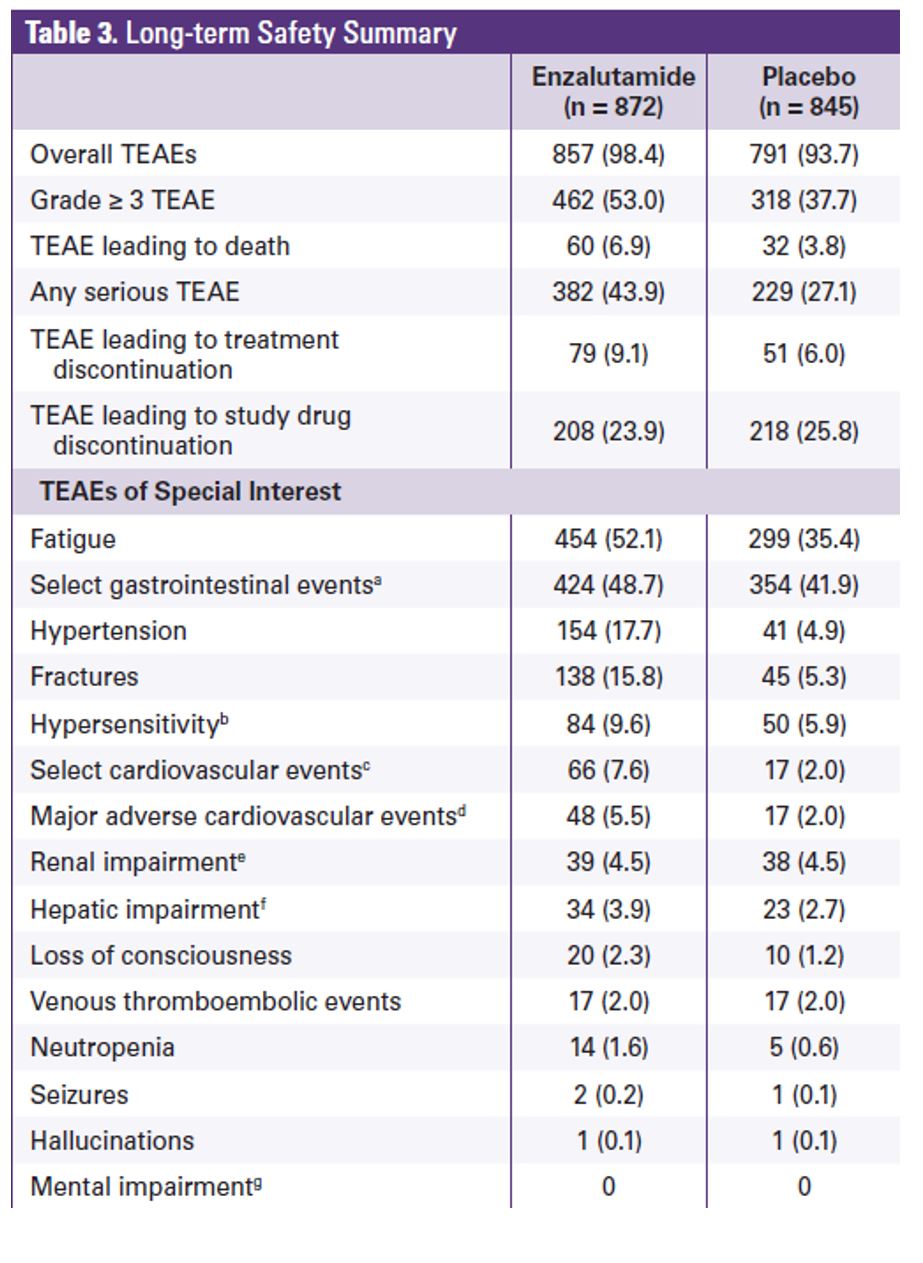
The enzalutamide treatment effect was generally consistent across all baseline disease specific subgroups, as shown in figure 3. At the data cutoff, 70% of the patients in the enzalutamide arm and 80% in the placebo arm had received >=1 postbaseline antineoplastic therapy. The most common subsequent therapy was docetaxel (enzalutamide arm 55%; placebo arm 62%) followed by abiraterone (enzalutamide arm 42%, and placebo arm 51%). Treatment emergent adverse effect was the primary reason for treatment discontinuation in 9.1% of the patients in the enzalutamide arm and 6% in the placebo arm (Table 2). Adverse effects that led to death were observed in 6.9% of patients in the enzalutamide arm and 3.8% in the placebo arm.
In conclusion, with > 5 years of follow-up, enzalutamide continued to demonstrate benefit compared with placebo in overall survival with asymptomatic or mildly symptomatic metastatic CRPC despite crossover in the placebo arm and multiple subsequent therapies. The overall survival benefit of enzalutamide was observed across all patient subgroups studied aside from the liver metastases subgroup with relatively few patients. The safety profile with enzalutamide was consistent with that reported previously.
Figure 3: Long term overall survival from PREVAIL by subgroup:

Table 2- Long term safety summary:
Presented by: Andrew Armstrong, MD, Duke University, Department of Medicine and Surgery, Durham, North Carolina
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 34th European Association of Urology (EAU 2019) #EAU19 conference in Barcelona, Spain from March 15-19, 2019.


