A total of 359 patients who underwent mpMRI of the prostate with subsequent fusion mpMRI-target biopsy and concomitant systematic biopsy, at a single European Centre between the years 2013 and 2018. All patients were either biopsy naïve or had a previous negative biopsy and all had a negative digital rectal examination. The study’s primary endpoint was clinically significant prostate cancer, defined as a biopsy Gleason score of ≥3+4 outside the index lesion. The authors also performed multivariable logistic regression analysis to develop a predictive model for the study’s primary outcome. Covariates in the model included biopsy age, PSA, prostate volume, PIRADS score, number of MRI-detected lesions and previous biopsy history. Multivariable-derived coefficients were used to develop a novel risk-calculator. External validation of the model was performed in a North American population of 462 patients, using the predetermined regression coefficient. The model was evaluated using the receiver operating characteristic-derived area under the curve (AUC), calibration plot, and decision-curve analyses (DCA) both in the development and validation cohort.
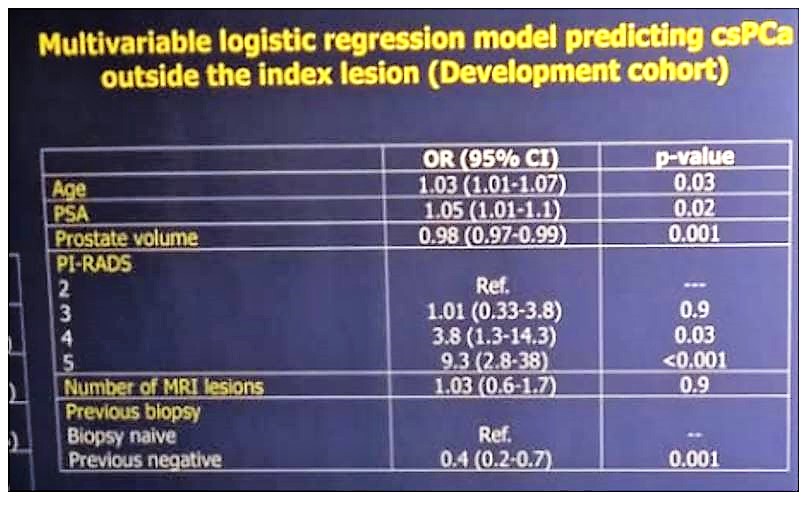
Table 1 demonstrates the basic clinical characteristics of the patients. Overall, the detection rate of clinically significant prostate cancer was 53.5%. specifically, for TRUS-systematic biopsy, it was 39.8%. In multivariable logistic regression analysis (Table 2), age at biopsy (OR: 1.03), PSA (OR: 1.05), prostate volume (OR: 0.98), previous negative biopsy (OR: 0.4), PIRADS 4 (OR: 3.8) and PIRADS 5 (OR: 9.3), were statistically significant independent predictors of clinically significant prostate cancer outside the index lesion.
Table 1 – Basic clinical characteristic of the patients:
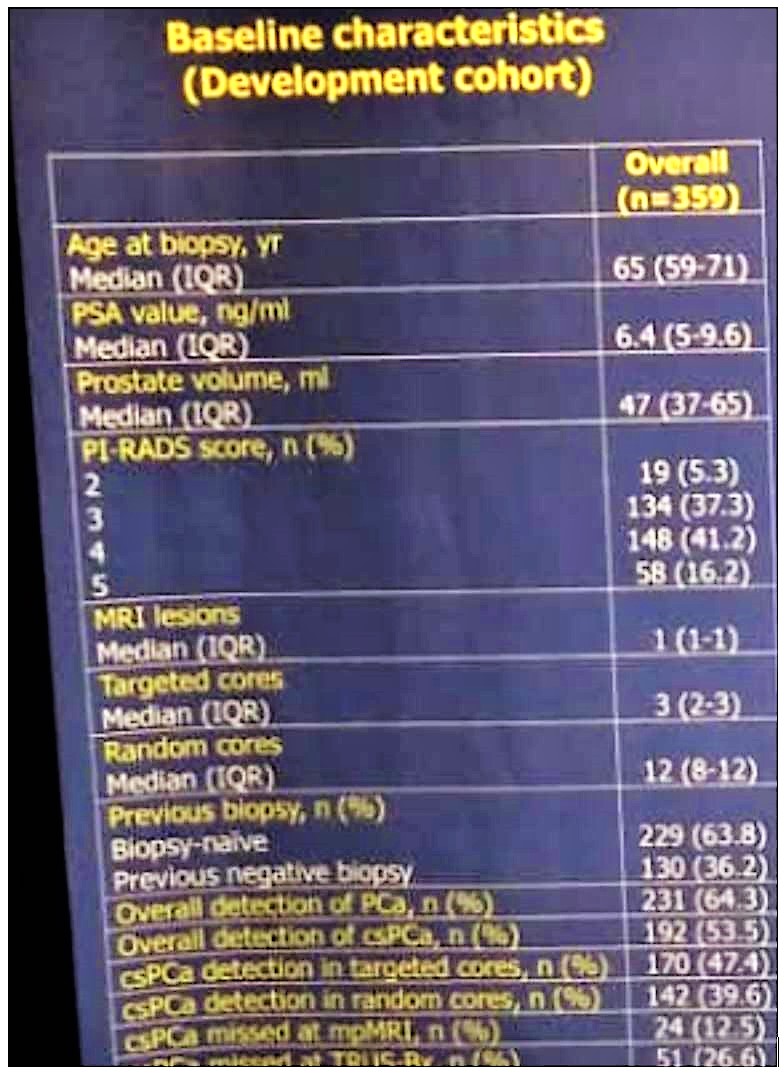
Table 2 – Multivariable logistic regression analysis to predict clinically significant
The multivariable model had an AUC of 0.78 and the calibration plot was good. External validation of the nomogram showed an AUC of 0.74 and the calibration plot was also good. The authors demonstrated that the application of this risk-calculator in the validation cohort would have spared 21.4% of TRUS-systematic biopsies at the cost of missing 5% of clinically significant cancer. Nonetheless, when evaluating the clinical utility of the model using decision curve analysis, no net benefit was observed for threshold probability investigated (<15%) compared to the standard of care (performing TRUS-systematic biopsy in addition to MRI-targeted biopsy in all patients), both in the development and validation cohort.
In conclusion, despite good accuracy, the multivariable model failed to demonstrate any clinical net benefit. Given the lack of clinical utility of this presented tool, the combination of MRI-targeted biopsy and TRUS-systematic biopsy should continue to be considered as the best possible approach to reduce the risk of missing clinically significant prostate cancer.
Presented by: Paolo Dell'Oglio, MD, IRCCS Ospedale San Raffaele, Division of Oncology, Unit of Urology, Milan, Italy
Written By: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 34th European Association of Urology (EAU 2019) #EAU19, conference in Barcelona, Spain from March 15-19, 2019.


