(UroToday.com) The 37th Annual European Association of Urology Congress held in Amsterdam, the Netherlands between July 1st, and 4th 2022 was host to an advanced bladder cancer abstract session about staging, predicting factors, and systemic therapy options. Dr. Taoka presented the results of his group’s study evaluating the association of prior treatment history for non-muscle invasive bladder cancer (NMIBC) on efficacy of pembrolizumab for patients with metastatic urothelial carcinoma (mUC).
Dr. Taoka began his presentation by noting that mUC patients with high PD-L1 expression levels benefit from PD-1/PD-L1 targeted therapy with improved survival outcomes. High expression of PD-L1 in BCG-resistant NMIBC has been shown. Currently, little is known about the impact of prior NMIBC treatment, including BCG usage, on the effectiveness of pembrolizumab. The authors hypothesized that genomic changes induced by prior NMIBC treatment including BCG may enhance the effectiveness of pembrolizumab in mUC patients.
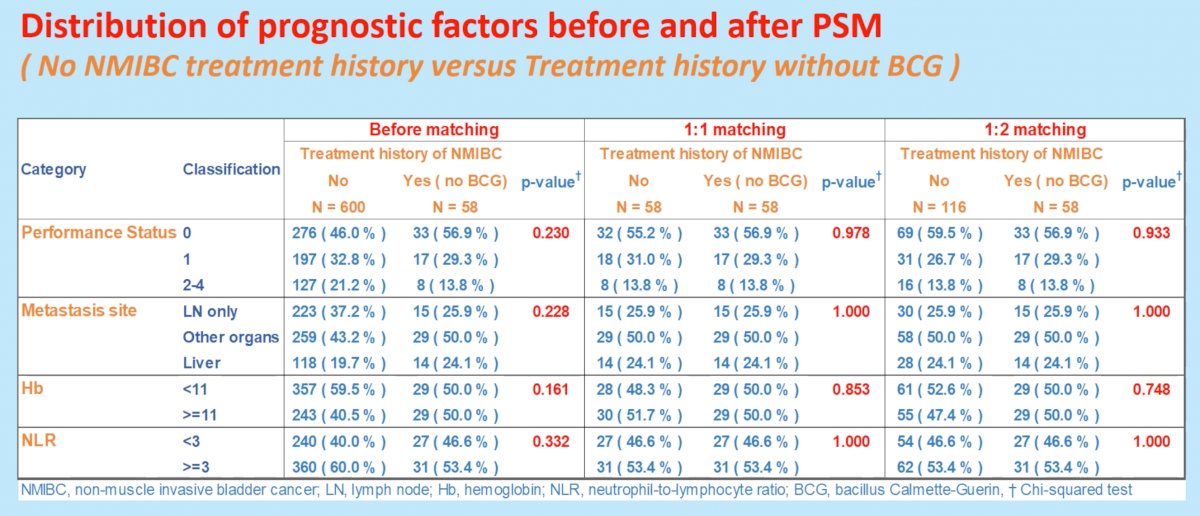
To this end, the authors retrospectively reviewed the clinicopathologic data of 755 patients with metastatic, chemo-resistant urothelial cancer who received pembrolizumab under the Japan Urological Oncology Group working group. Of these 755 patients, 600 had no treatment history of NMIBC. Of the remaining 155 with history of NMIBC, 97 had a history of BCG usage and 58 did not.
The overall response rate (ORR), disease control rate (DCR) and overall survival (OS) from the initiation of pembrolizumab were analyzed by history of NMIBC treatment, including the use of BCG, using propensity score matching with four covariates (performance status, metastatic site, hemoglobin, neutrophil to lymphocyte ratio).
Baseline clinical parameters were well balanced after performing propensity score matching between the initial two groups of interest: (i) No treatment history of NMIBC and (ii) treatment history of NMIBC (without BCG).
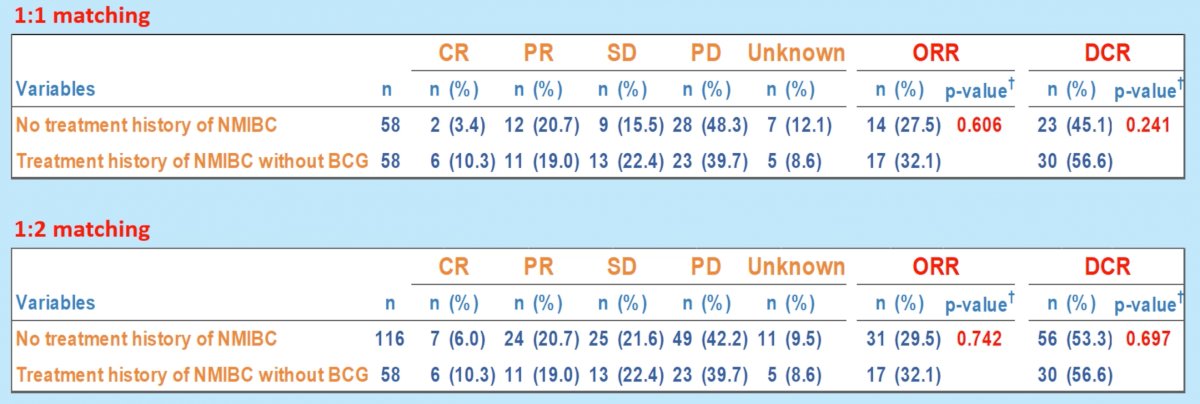
There were no significant differences in ORR and DCR between the two groups of interest, regardless of whether 1:1 or 1:2 propensity score matching was performed.
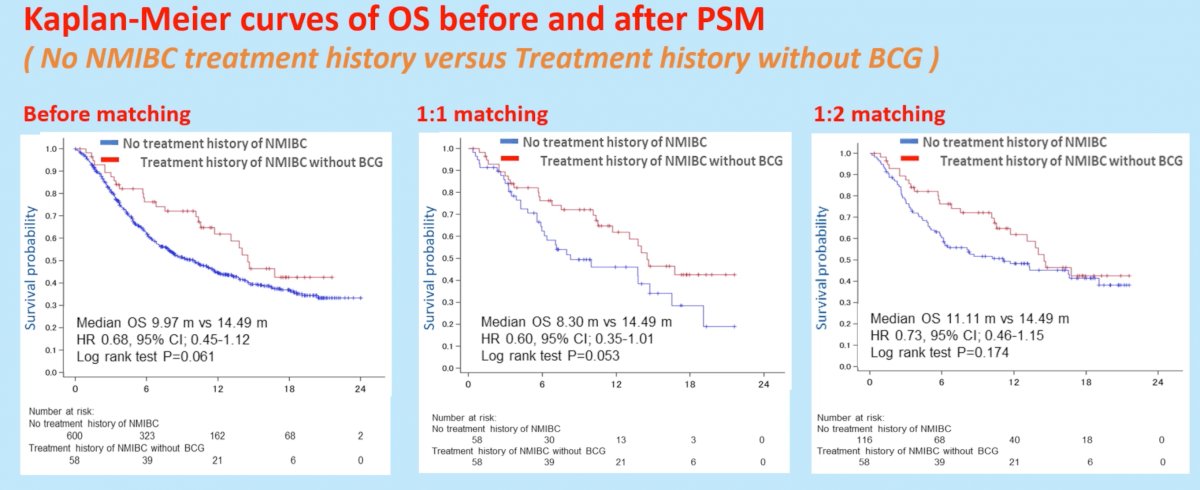
With regards to OS, there appeared to be no significant differences in OS between the two arms. With 1:1 matching, median OS was 8.30 months for patients with no treatment history of NMIBC, compared to 14.49 months in those with treatment history without BCG for NMIBC (HR: 0.60, 95% CI: 0.35-1.01, p=0.053).
Similar analysis was next performed comparing patients (i) without history of NMIBC treatment and (ii) those with history of NMIBC treatment (with BCG).
Propensity score matching similarly achieved balance between the two groups with regards to performance status, metastatic sites, hemoglobin levels, and neutrophil to lymphocyte ratios.

In contrast to the previous comparisons between patients with no prior history of NMIBC and those with history of NMIBC treatment without BCG, comparisons after 1:1 propensity score matching between those without history of NMIBC and those with treatment history with BCG demonstrated superior ORR in patients with no history of NMIBC treatment (33.3%) compared to patients with prior BCG treatment for NMIBC (19.8%, p=0.042). There were no significant differences in DCR: 54.4% and 55.8%, respectively (p=0.855).
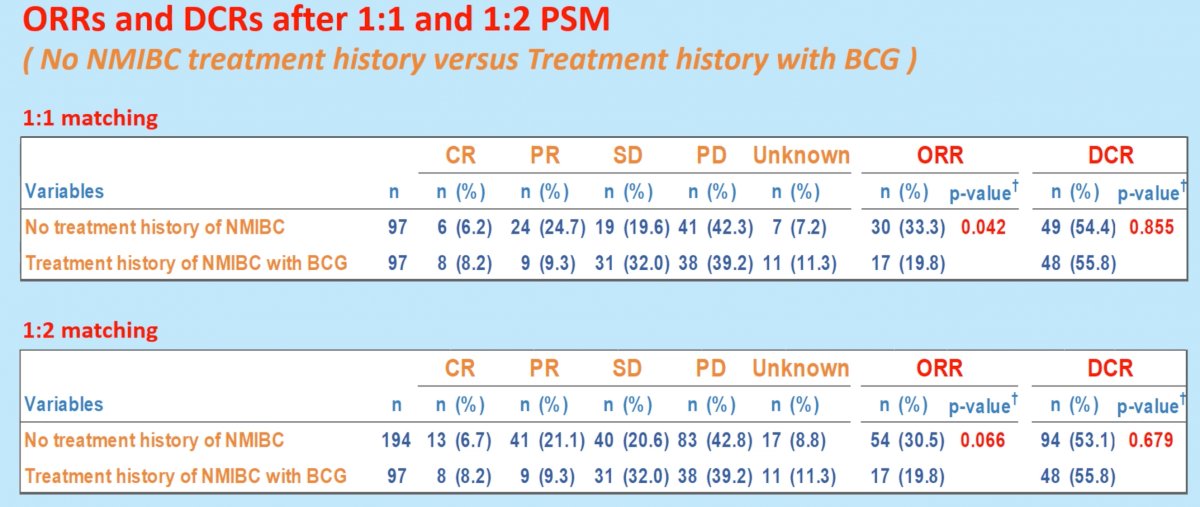
These differences in ORR did not translate into significant OS differences, whereby 1:1 matching demonstrated OS rates of 10.1 months in those without history of NMIBC and 12.1 months in those with history of NMIBC treatment with BCG (HR 0.92, p=0.66).
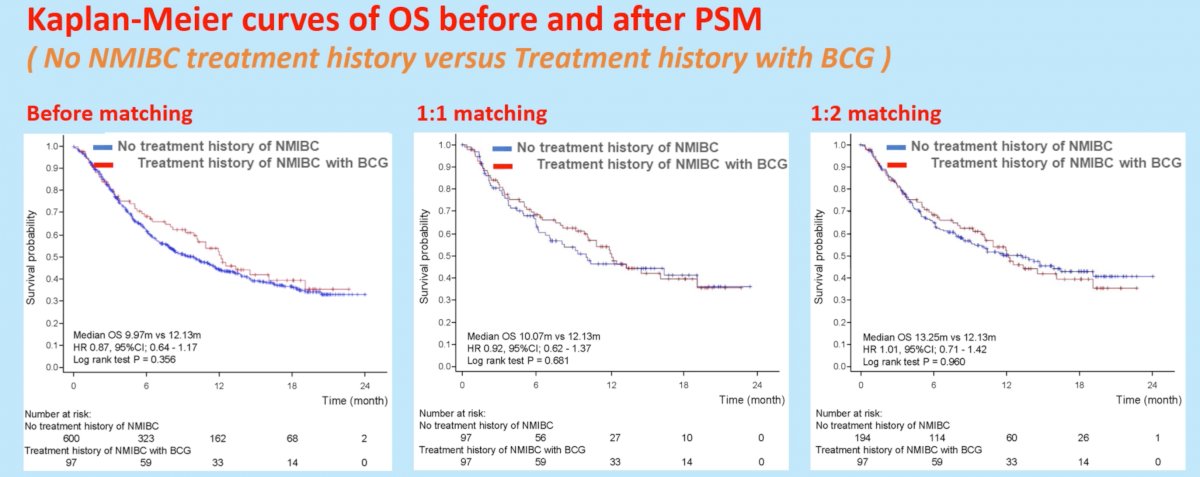
The authors concluded that this study analyzing real-world data using propensity score matching did not find any association between prior NMIBC treatment, including BCG, and the effectiveness of pembrolizumab for chemo-resistant mUC
Presented by: Dr. Rikiya Taoka, MD, PhD, Department of Urology, Kagawa University, Takamatsu, Japan
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2022 European Association of Urology (EAU) Annual Hybrid Meeting, Amsterdam, NL, Fri, July 1 – Mon, July 4, 2022.


