(UroToday.com) The 37th Annual European Association of Urology Congress held in Amsterdam, the Netherlands between July 1st, and 4th 2022 was host to an advanced bladder cancer abstract session about staging, predicting factors, and systemic therapy options. Dr. Roberto Iacovelli presented results of the ARIES trial evaluating first line avelumab in cisplatin-ineligible, Programmed Cell Death Ligand 1 (PD-L1) positive patients with metastatic or locally advanced urothelial carcinoma (aUC).
Dr. Iacovelli began his presentation by noting that cisplatin-based chemotherapy remains the recommended first line treatment for eligible patients with aUC. Almost half of aUC patients are deemed cisplatin-ineligible, with some also considered platinum ineligible. Furthermore, cisplatin-ineligible patients have poor prognoses and frequently have higher comorbidity disease burdens.
Avelumab is a human immunoglobulin G1 (IgG1) monoclonal antibody directed against PD-L1 protein. Avelumab may restore immune function through the activation of cytotoxic T-lymphocytes targeted to PD-L1-overexpressing tumor cells. Additionally, avelumab induces an antibody-dependent cellular cytotoxic response against PD-L1-expressing tumor cells.
Results from the JAVELIN-100 trial have demonstrated that avelumab maintenance improves overall survival (OS) and progression-free survival (PFS) in patients with aUC with stable or responsive disease after first line platinum-based chemotherapy.1 The ARIES trial aimed to investigate the efficacy and safety of avelumab in aUC patient unfit for cisplatin and PD-L1 positive status.
A study sample size of 67 was required to show an improvement in 1-year OS rate from 40% to 57% with 80% error and an alpha error of 0.05.
198 patients were evaluated, of whom 71 had PD-L1 expression >=5%. These patients received avelumab 10 mg/kg every 2 weeks. The study endpoints were:
- Primary: 1-year OS rate
- Secondary: Median OS, median PFS, overall response rate, safety, quality of life measures, and outcome based on PD-L1 expression
The baseline patient characteristics are displayed below. Notably, 35 (49.3%) had visceral disease and the most common reason for cisplatin-ineligibility was Cr clearance <60 ml/min (70.4%) and ECOG performance status of 2 (31.0%).
As of data cut off of February 2nd 2022, the median follow up was 10.0 months and 14 (19.9%) patients remained on treatment. The median OS was 10.0 months (95% CI: 5.5 – 14.5) and the 1-year OS rate was 43.0%. The median PFS was 2.0 months (95% CI: 1.7-2.3).
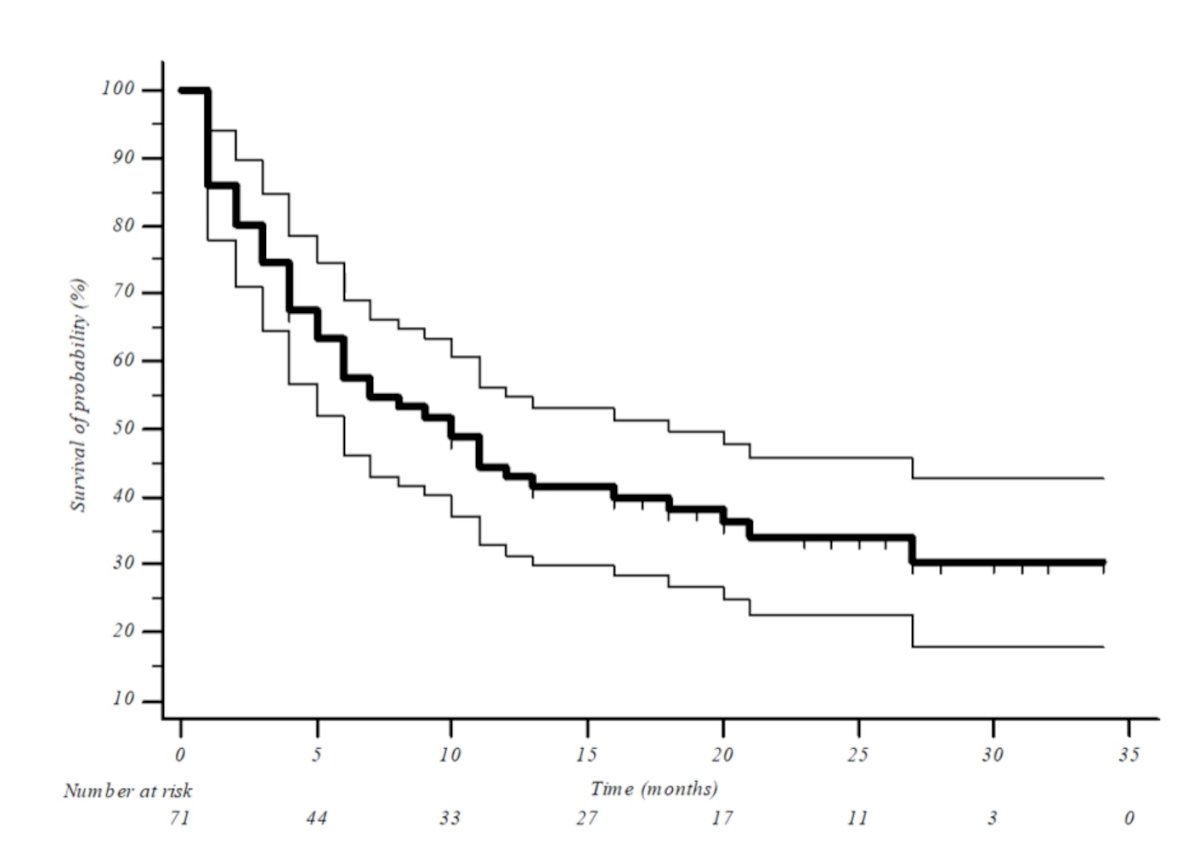
With regards to response rate, avelumab achieved a disease control rate of 43.7%, with complete and partial responses seen in 6 (8.5%) and 11 (15.5%) patients, respectively.

Any grade adverse events were reported in 67 (94.4%) patients and high-grade adverse events in 30 (42.3%) patients. Two patients (2.8%) experienced fatal adverse events.
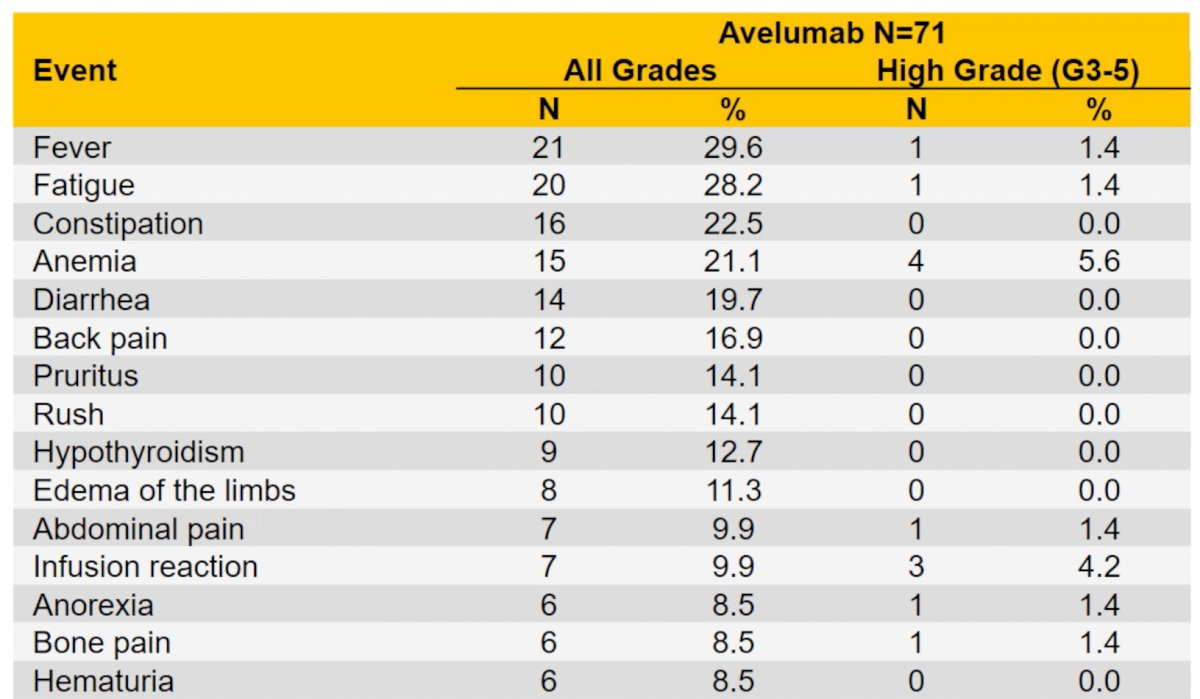
Dr. Iacovelli concluded as follows:
- First line avelumab demonstrates activity in cisplatin-ineligible aUC patients with a notable complete response rate of 8.5% and a disease control rate of 43.7%
- The ARIES trial confirmed the favorable safety profile of avelumab in aUC
- Avelumab was unable to improve 1-year OS as expected (actuarial 43.0% versus expected 57.0%)
- The response rate and median OS reported in the ARIES trial were comparable with other available evidence in the literature
- Patients with aUC unfit for cisplatin have a dismal prognosis. Clinical selection is critical to maximize efficacy of available therapeutic options including immunotherapy.
Presented by: Dr. Roberto Iacovelli, MD, PhD, Department of Medical Oncology, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2022 European Association of Urology (EAU) Annual Hybrid Meeting, Amsterdam, NL, Fri, July 1 – Mon, July 4, 2022.
References:


