(UroToday.com) The 2022 EAU annual meeting featured a joint session of the EAU, EANM, ESMO, and ESTRO societies examining modern diagnostic and therapeutic approaches in prostate cancer, including a presentation by Dr. Matthias Eiber discussing the role of PSMA radioligand treatment in castrate-resistant M1 prostate cancer. Dr. Eiber notes that the short history of PSMA radioligand therapy dates back to 2012 and recently culminated earlier this year with the FDA approval of Pluvicto:
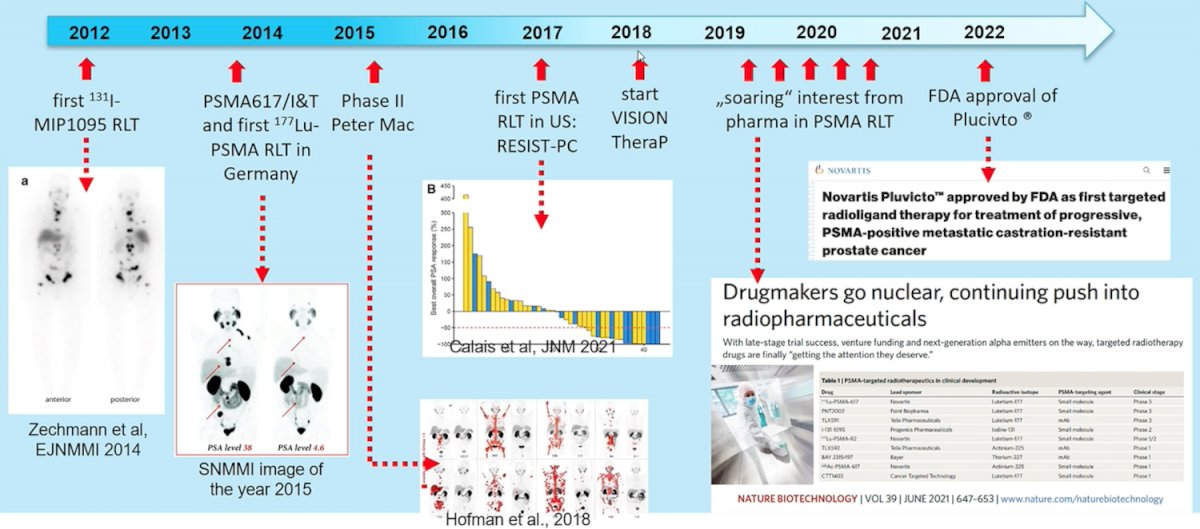
In 2018, the phase II LuPSMA trial was published,1 assessing the utility of 177Lu-PSMA-617 among 30 patients with mCRPC that had progressed after standard treatments, including taxane-based chemotherapy and second-generation anti-androgens, at the Peter MacCallum Cancer Centre in Melbourne, Australia. Overall, 17 (57%) of 30 patients (95% CI 37-75) achieved a PSA decline of 50% or more, and there were no treatment-related deaths:
Additionally, objective response in nodal or visceral disease was reported in 14 (82%) of 17 patients with measurable disease. The most common toxic effects related to 177Lu-PSMA-617 were grade 1 dry mouth recorded in 26 (87%) patients, grade 1 and 2 transient nausea in 15 (50%), and grade 1-2 fatigue in 15 (50%). Grade 3 or 4 thrombocytopenia possibly attributed to 177Lu-PSMA-617 occurred in four (13%) patients.
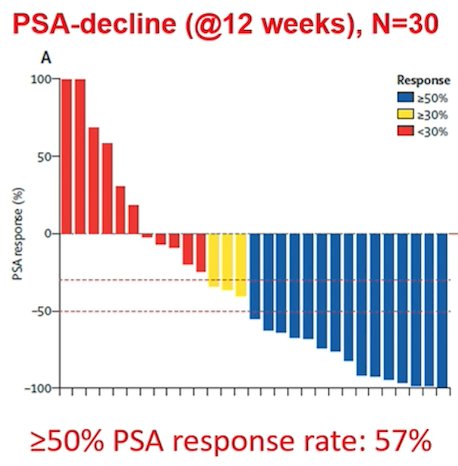
The phase 2 177Lu-PSMA-617 trial set the stage for the phase 3 VISION trial, published in 2021.2 The VISION trial evaluated 177Lu-PSMA-617 in men with PSMA-positive mCRPC who had previously received treatment with next-generation androgen receptor signaling inhibition (abiraterone, enzalutamide, etc) and one or two prior lines of taxane chemotherapy. Importantly, patients must have had PSMA-positive disease on the basis of a central review of 68Ga-PSMA-11 staging scans. PSMA positivity was defined as uptake greater in metastatic lesions than in the liver. Further, they could have no PSMA-negative metastatic lesions.
Following enrollment, patients were randomized in a 2:1 fashion to receive either 177Lu-PSMA-617 (7.4 GBq every 6 weeks x 6 cycles) plus standard of care or standard of care alone. Standard of care treatments were at the discretion of the treating investigator; however, cytotoxic chemotherapy, immunotherapy, and radium-223 were explicitly excluded. Most patients received alternative androgen-directed therapies while others received palliative radiotherapy and glucocorticoids. The trial schema for VISION is as follows:
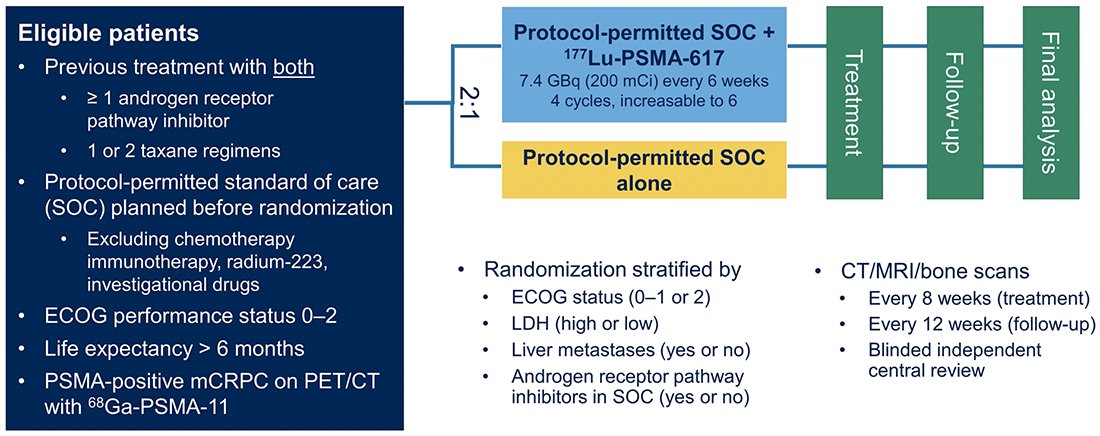
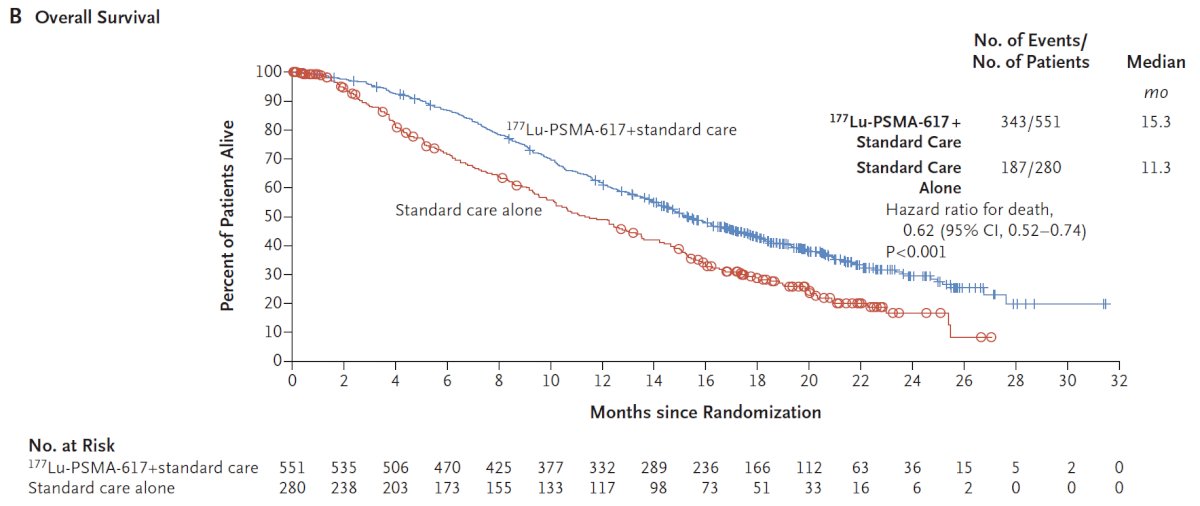
The trial assessed two alternate primary endpoints: radiographic progression-free survival using PCWG3 criteria by independent central review and overall survival. Among 1,179 screened patients, the VISION trial enrolled 831 patients, including 551 patients were allocated to 177Lu-PSMA-617 + standard of care and 280 were allocated to standard of care only. In both the radiographic progression free survival analysis set and among all randomized patients, between 45-50% of patients had received more than one androgen receptor pathway inhibitor and 41-48% of patients had received more than one taxane regime. Over a median study follow-up of 20.9 months, treatment with 177Lu-PSMA-617+ standard of care significantly improved overall survival by a median of 4.0 months (median overall survival, 15.3 vs 11.3 months; HR 0.62, 95% CI 0.52, 0.74), compared to standard of care alone, in the overall cohort of all randomized patients (n=831):
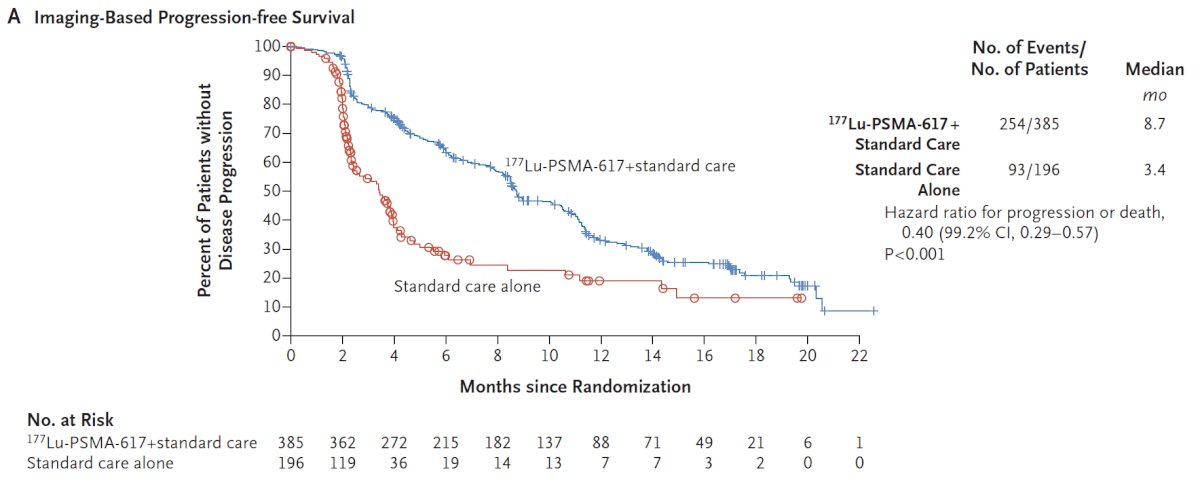
The second alternate primary endpoint showed that treatment with 177Lu-PSMA-617 + standard of care significantly improved radiographic progression free survival by a median 5.3 months (median radiographic progression free survival, 8.7 vs 3.4 months; HR 0.40, 99.2% CI 0.29, 0.57):
While a higher rate of high-grade (grade 3-5) treatment-emergent adverse events was observed with 177Lu-PSMA-617 (28.4% vs 3.9%), overall therapy was well tolerated. In terms of specific adverse events, treatment with 177Lu-PSMA-617 + standard of care was associated with increased rates of bone marrow suppression, xerostomia, and nausea and vomiting.
The TheraP trial was the first randomized trial of LuPSMA versus cabazitaxel.3 This trial enrolled patients with mCRPC who had previously received docetaxel and were eligible to receive cabazitaxel. Patients were required to have progressive disease with a rising PSA with absolute PSA of 20 ng/mL or higher. All patients underwent both Ga-68-PSMA-PET/CT and F-18-FDG-PET/CT prior to randomization. To be eligible for inclusion, patients must have had a high avidity lesion on PSMA PET/CT (SUV max >20 at any site) with measurable disease with SUV max of 10 or greater. Further, there could be no sites of disease which were FDG positive but PSMA negative.
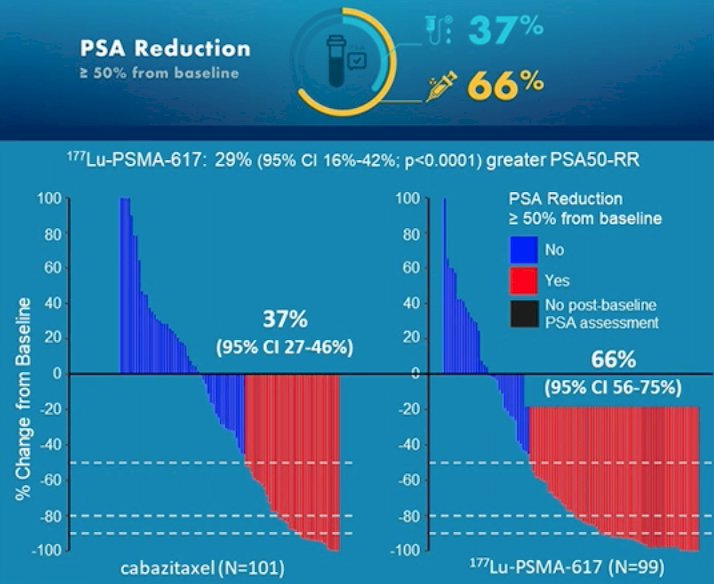
Among 200 men at 11 sites in Australia who were eligible, randomization was performed in a 1:1 fashion to 177Lu-PSMA-617 or cabazitaxel. The primary study outcome was PSA response: compared to those receiving cabazitaxel (37%, 95% CI 27 to 46%), responses were significantly higher among those who received Lu-PSMA (66%, 95% CI 56 to 75%) with an absolute difference of 29% (95% CI 16 to 42%, p<0.0001):
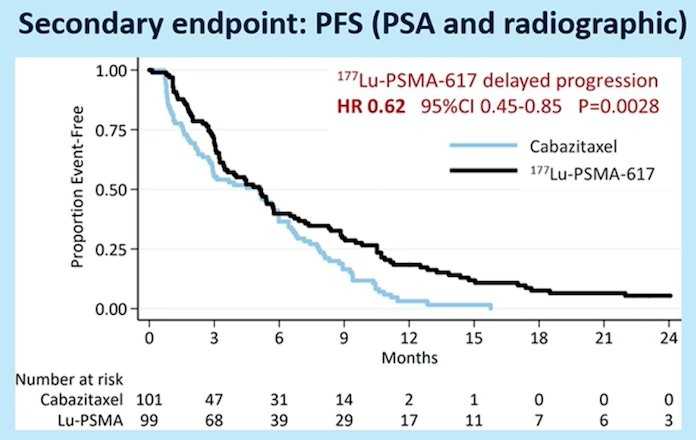
More recent data were presented at ASCO-GU 2021 examining secondary endpoints including PSA/radiologic PFS (PCWG3) and overall survival. PFS was significantly longer in those assigned Lu-PSMA rather than cabazitaxel (rates at 1 year 19% [95% CI 12-27%] vs 3% [95% CI 1-9%], HR 0.62, 95% CI 0.45-0.85):
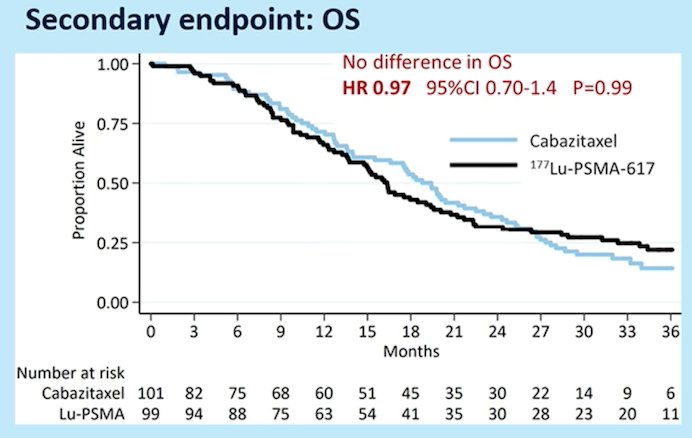
As similar benefit was seen whether PFS was examined radiographically (rPFS, HR 0.64, 95% CI 0.46-0.88; p = 0.007; 160 events) or based on PSA (PSA-PFS, HR 0.60 95% CI 0.44-0.83; p = 0.002; 172 events). Currently, after 36 months of follow-up, there is no benefit in overall survival (HR 0.97, 95% CI 0.70-1.40):
Dr. Eiber notes that there is debate regarding whether we should image with PSMA or PSMA + FDG-PET for radioligand selection. PSMA PET was routinely used for screening prior to LuPSMA, but there is no consensus was to what is “adequate uptake.” Currently, there are several approaches used to determine adequate uptake:
- Technical University of Munich approach (“routine clinical use”): higher than liver uptake, no PSMA-negative visceral disease/large soft tissue lesions (estimated 5-10% of patients are ineligible)
- VISION approach: PSMA-uptake greater than the liver in at least one lesion plus in all lymph nodes >= 2.5 cm/all visceral lesions >= 1cm/extraosseous soft tissue bone components (12.6% of patients are ineligible)
- Hofman et al 2018 approach: at dominant tumor sites SUVmax 1.5x the SUVmean of the liver, with no FDG discordance (16.3% of patients are ineligible)
- TheraP approach: PSMA SUVmax > 20 at any site, measurable sites SUVmax > 10, no PSMA negative/FDG positive disease (27.5% of patients are ineligible)
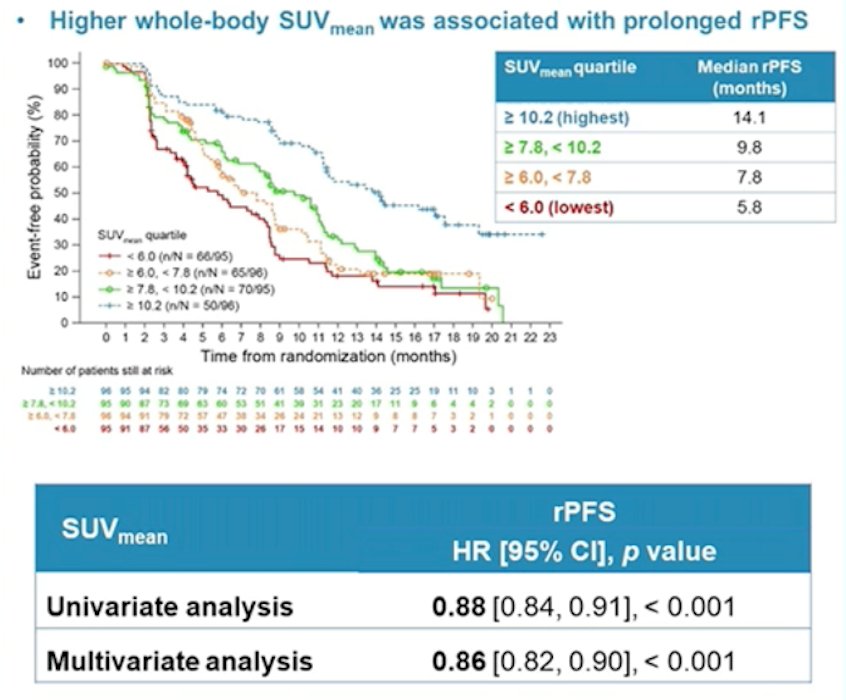
Several small post-hoc analyses have assessed eligibility for PSMA radioligand therapy. In an assessment of 301 patients that underwent radioligand therapy, 29 patients were not eligible based on the VISION criteria, with these patients have less of a PSA50 decline (21% versus 50%, p = 0.0005) and a trend towards shorter median overall survival (9.6 versus 14.2 months, p = 0.16). In a post-hoc analysis of the VISION study presented at ASCO 2022, higher whole-body SUVmean was associated with improved clinical outcomes: those patients in the highest quartile (SUVmean: rPFS, ≥ 10.2; OS, ≥ 9.9) had a median rPFS and OS of 14.1 and 21.4 months, vs 5.8 and 14.5 months for those in the lowest quartile (< 6.0; < 5.7), respectively:
Dr. Eiber concluded his presentation by discussing the role of PSMA radioligand treatment in castrate-resistant M1 prostate cancer with the following take-home messages:
- 177Lu-PSMA is safe and shows an overall survival benefit in mCRPC patients treated with 3+ lines of therapy (FDA approval March 2022, EMA approval expected)
- Imaging selection seems critical but questions remain regarding implementation
- Alpha emitter radioligand therapy is currently being explored, however there is associated significant xerostomia
- Use in earlier stages and combination treatments are currently being explored
Presented by: Matthias Eiber, MD, PhD, Department of Nuclear Medicine, Technical University of Munich, Munich, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Association of Urology (EAU) Annual Hybrid Meeting, Amsterdam, NL, Fri, July 1 – Mon, July 4, 2022
References:
- Hofman MS, Violet J, Hicks RJ, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-center, single-arm phase 2 study. Lancet Oncol 2018 Jun;19(6):825-833.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021 Feb 27;397(10276):797-804.


