(UroToday.com) The 2022 EAU annual meeting featured a joint session of the EAU, EANM, ESMO, and ESTRO societies examining modern diagnostic and therapeutic approaches in prostate cancer, including a presentation by Dr. Elena Castro discussing the current landscape of treatment for castrate resistant M1 prostate cancer. Dr. Castro notes that the current treatment landscape for mCRPC is as follows, but also highlights that this algorithm is based on conventional imaging modalities:
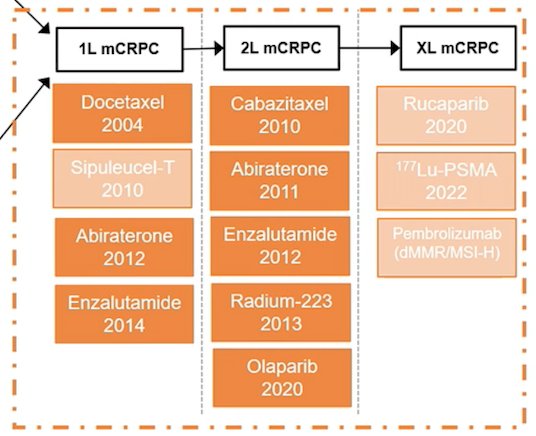
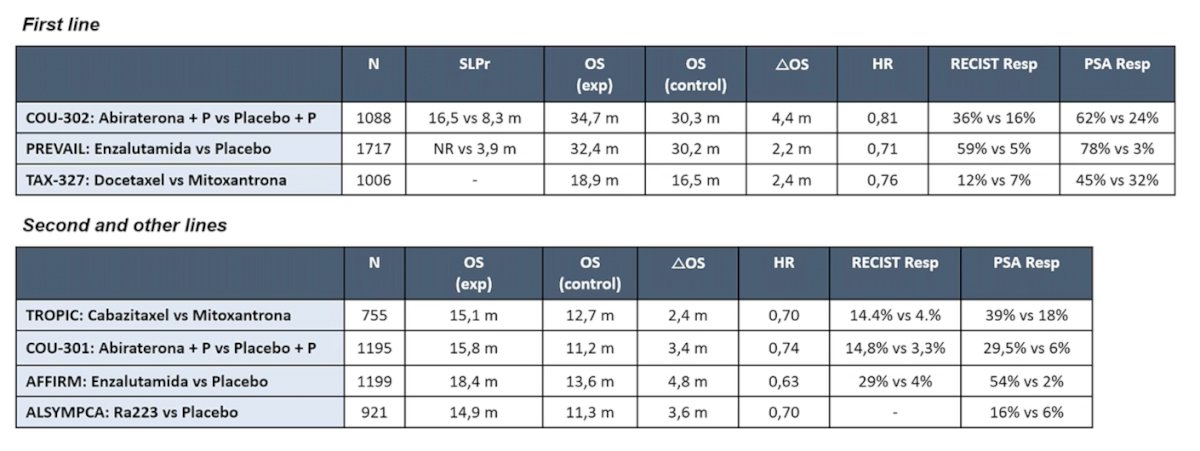
As such, trials that were conducted in the past may not represent the mCRPC population in 2022. Previously published first line trials included COU-302, PREVAIL, and TAX-327, whereas second line trials included TROPIC, COU-301, AFFIRM, and ALSYMPCA:
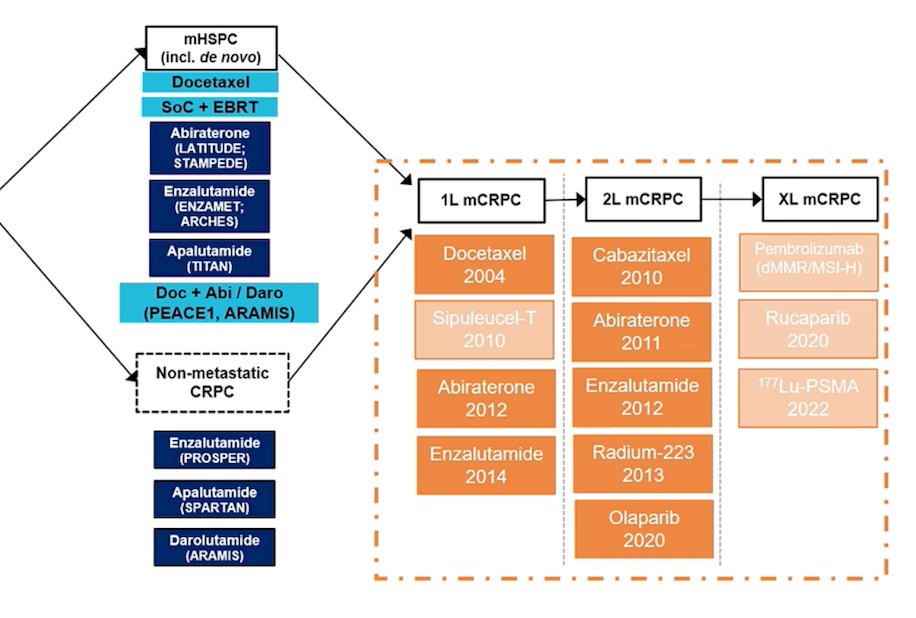
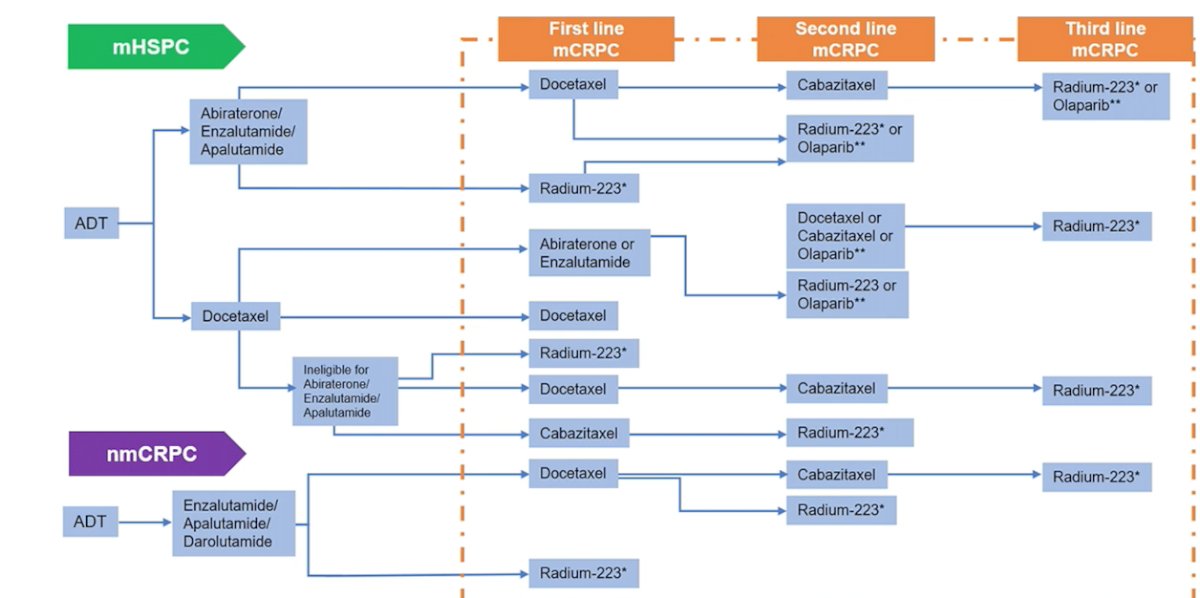
Broadening out the treatment landscape to advanced prostate cancer (including mHSPC and non-metastatic CRPC), Dr. Castro notes that the treatment landscape is as follows:
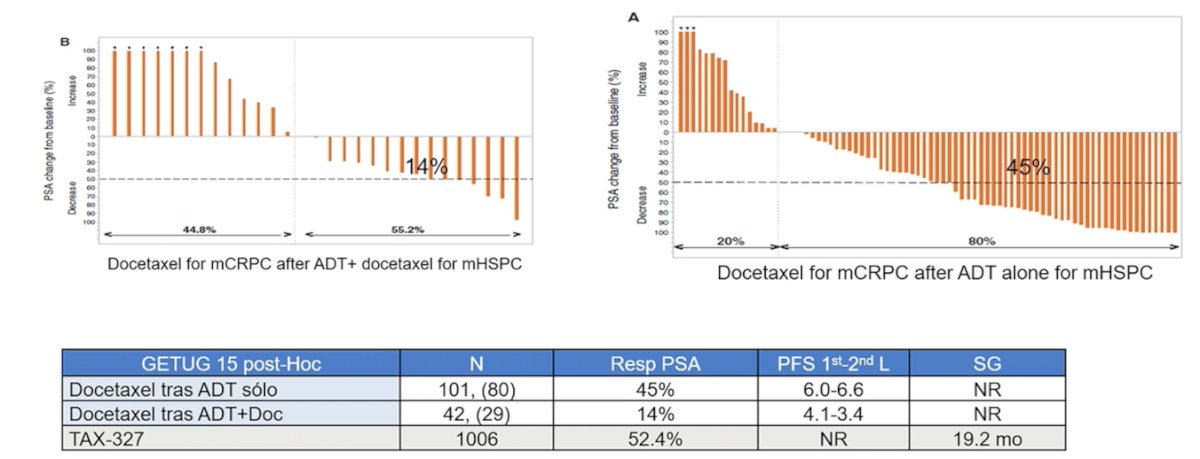
Dr. Castro made the important point that how we treat patients in the mCRPC state depends on what early systemic treatment the patient received. Previous studies suggest that patients that receive a docetaxel rechallenge in the mCRPC state after having received docetaxel in the mHSPC state have a poorer PSA response compared to those receiving docetaxel for mCRPC after ADT alone in mHSPC:
Additionally, there is cross-resistance between the current second-generation androgen receptor inhibitors in mCRPC, which has implications on treatment sequencing. Previous work has shown that pre-treatment with enzalutamide results in limited response to both abiraterone and combination second-generation androgen receptor inhibitors. Based on the impact of previous therapies received, Dr. Castro provides the following proposed treatment algorithm for mCRPC therapy, with the likelihood of this changing quite quickly in the future after implementation of PSMA radioligand therapy:
Importantly, the biology of tumors changes in response to treatments, particularly given that lineage plasticity has emerged as a mechanism of resistance to androgen receptor-targeted therapy. There are several key components of aggressive variant prostate cancer, which has historically been associated with a very poor prognosis. Aggressive variant disease is defined as mCRPC with at least one of the following:
- Histologic evidence of small-cell prostate carcinoma (pure or mixed)
- Exclusively visceral metastases
- Radiographically predominant lytic bone metastases by plain x-ray or CT scan
- Bulky lymphadenopathy or a bulky high-grade (Gleason >= 8) tumor mass in the prostate/pelvic
- Low PSA (<= 10 ng/mL) + high volume (>= 20) bone metastases (“low PSA secretors”)
- Neuroendocrine markers on histology or in serum + elevated LDH, CEA or malignant hypercalcemia
- Short interval to ADT progression following the initiation of hormonal therapy
- Enriched for deletions and/or mutations in at least 2 or 3 TP53, RB1, and PTEN
Over the last several years, precision medicine for advanced prostate cancer has emerged, identifying actionable alterations detectable in most mCRPC patients, including the AR pathway (70%), PI3K-AKT-mTOR pathway (40-60%), DDR mutations (25%), cell cycle regulators RB1/CDK (25%), and others. The current scenario of PARP inhibitors by HRR alterations in mCRPC is as follows:
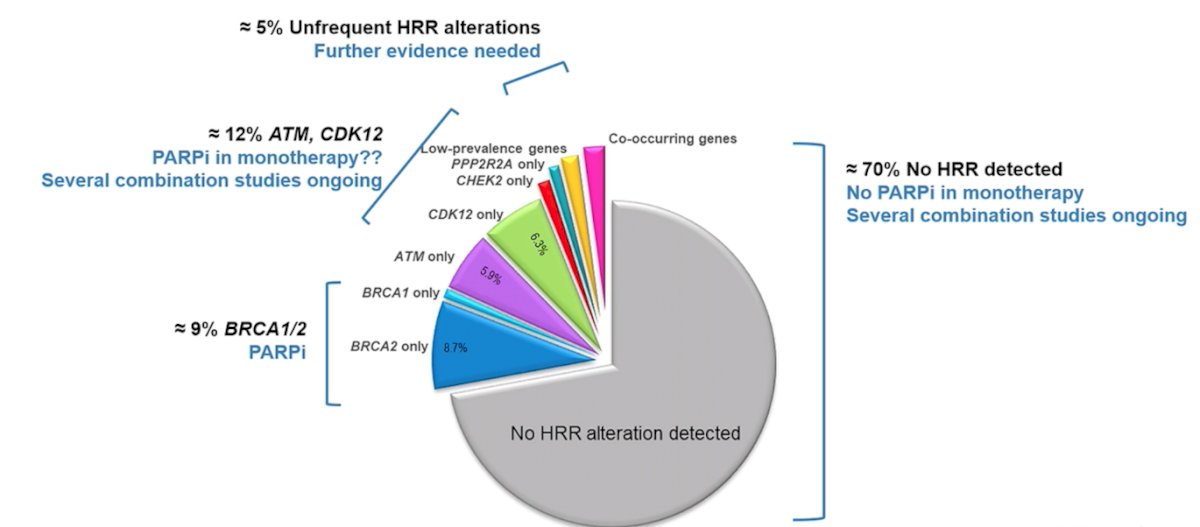
Genomic alterations in mCRPC that are currently targetable include:
- BRCA1/2 alterations PARP inhibitors (olaparib, rucaparib, niraparib, talazoparib)
- HRR loss of function PARP inhibitors (olaparib, rucaparib, niraparib, talazoparib)
- DDR (HRR) alterations platinum-based chemotherapy
- dMMR/MSI-H/TMB >10 anti PD1/PDL1
- PI3K pathway alterations AKT inhibitors (ipatasertib, capivasertib)
Dr. Castro emphasized that one of the main issues is quality of tissue for tumor genomic profiling. In the PROfound trial [1], 31% of samples were inadequate for genomic profiling, 32% were inadequate in TRITON2 [2], and 33% were not evaluable in IPATential150. Sample selection and optimization of tissue collection is critical, since 30%-50% of prostate cancer samples fail next generation sequencing.
Dr. Castro concluded her presentation discussing the current landscape of treatment for castrate resistant M1 prostate cancer by emphasizing that based on recently presented data at ASCO 2022, only 50% of patients in the real-world setting are receiving more than first-line treatment for mCRPC. The main reasons for failure to provide subsequent lines of therapies remains to be fully understood.
Presented by: Elena Castro, MD, Department of Medical Oncology, Instituto de Investigacion Biomedica de Malaga, Malaga, Spain
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Association of Urology (EAU) Annual Hybrid Meeting, Amsterdam, NL, Fri, July 1 – Mon, July 4, 2022.
References:
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020 May 28;382(22):2091-2102.
- Abida W, Patnaik A, Campbell D, et al. Rucaparib in Men with Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J Clin Oncol 2020 Nov 10;38(32):3763-3772.


