(UroToday.com) The 2022 EAU annual meeting featured a game-changing session, including a presentation by Dr. Declan Murphy discussing initial results of LuTectomy, a single-arm study of the dosimetry, safety, and potential benefit of 177Lu-PSMA-617 prior to radical prostatectomy. Dr. Murphy notes that in patients with high-risk localized prostate cancer, we need to do better. Biochemical recurrence rates remain high and previous neoadjuvant strategies have failed to improve outcomes in these patients. Thus, the standard of care, surgery or radiotherapy, remains unchanged as we wait for these patients to experience biochemical recurrence. Over the last several years, LuPSMA has represented exciting progress in mCRPC. The first prospective data was published in 2018, demonstrating that the treatments were well tolerated and had excellent responses. TheraP1 and VISION2 have confirmed the benefits of 177Lu-PSMA-617 in mCRPC, so is it possible that there could be a role in localized disease?
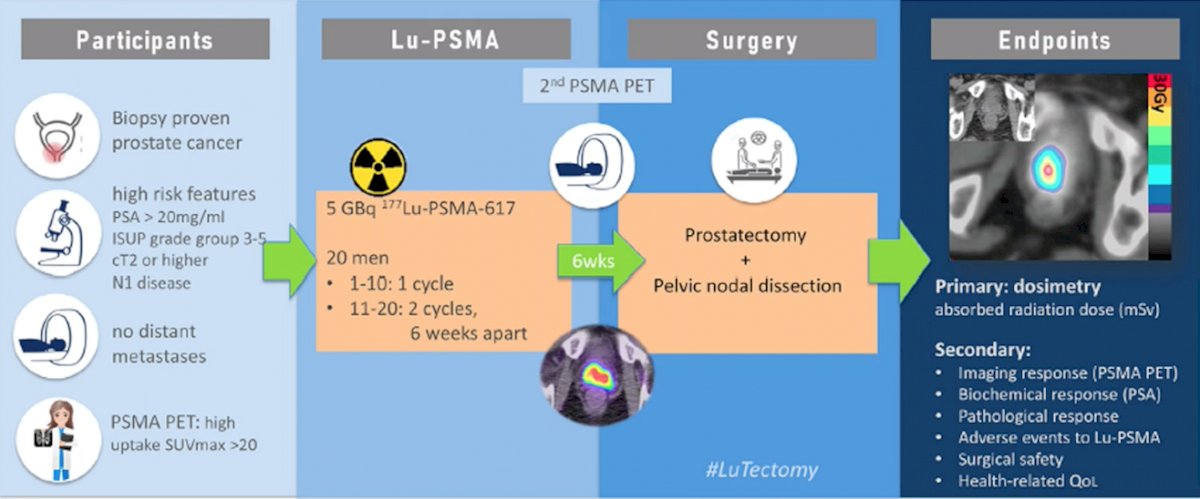
LuTectomy is a prospective phase I/II study of 177Lu-PSMA-617 prior to surgery. Patients have biopsy proven prostate cancer, with high features including: PSA > 20 ng/mL, ISUP grade group 3-5 disease, >= cT2 disease, or N1 disease. Patients must not have distant metastasis and a PSMA PET/CT must have high uptake SUVmax > 20. Patients then receive one (first ten patients) or two (second ten patients; cycles 6 weeks apart) of cycles of 5 GBq 177Lu-PSMA-617 followed by radical prostatectomy + pelvic nodal dissection. The primary endpoint is dosimetry (absorbed radiation dose mSv) and secondary endpoints include: imaging response (PSMA PET/CT), biochemical response, pathological response, adverse events to 177Lu-PSMA-617, surgical safety, and health-related quality of life.
The median patient age was 65 years, median PSA was 16 ng/mL (IQR 8-26), six patients were grade group 3 (four were grade group 4 or 5), 4 patients were cN1, 9 patients had PI-RADS 4-5 lesions on mpMRI, and the median SUVmax was 29 (IQR 27-39). The primary endpoint of absorbed radiation dose demonstrated a clinically meaningful dose absorbed by the prostate (median 48 Gy) and lymph nodes (median 50 Gy):

177Lu-PSMA-617 was also very well tolerated, demonstrating minimal organ dosimetry and no grade 2-5 adverse events:

Dr. Murphy also noted that the surgery was straightforward, with a median operative time of 190 minutes (IQR 180-208), mean estimated blood loss of 175 mL (range 112-300), 90% of patients staying in the hospital 1 day, and 5 patients having a Clavien 1-2 complication (no Clavien 3-5):

As follows is a case example of a 57 year old man with a PSA of 38 ng/mL, cT2b, PI-RADS 5 lesion on MRI, biopsy grade group 5, cN1, with a SUVmax of 25.1. After a single dose of 177Lu-PSMA-617, his prostate had an absorbed dose of radiation of 33 Gy and the lymph node on post-177Lu-PSMA-617 imaging had resolved (PSA decrease to 29 ng/mL). There were minimal radiation changes during his surgery and he had a final pathology of pT3a, grade group 5, negative surgical margins, and PSA < 0.2 at 18 months:

With regards to the secondary endpoint of imaging response, no patients had a complete PSMA response, 60% had a partial PSMA response, 30% had stable PSMA disease, and 1 patient had progressive PSMA disease. For the secondary endpoints of PSA response and biochemical recurrence, 80% of patients had a reduction in PSA levels, the median PSA reduction was 43% (IQR 62-29), the PSA50 response rate was 40%, and at a median follow-up of 12 months, the BCR-free survival was 80%. On pathological examination, eight men had evidence of treatment effect with no patients having a complete pathological response or minimal residual disease:

Dr. Murphy concluded his presentation dby iscussing the initial results of the LuTectomy trial with the following take-home messages:
- 177Lu-PSMA-617 prior to surgery is safe and significant doses of radiation can be delivered to the target tissues
- Encouraging biochemical, imaging, and pathological responses in these first 10 patients have been reported
- Thus far, there are no clinically meaningful oncological benefits in this early experience
- The second 10 patients (2 cycles of 177Lu-PSMA-617) have been recruited and results are expected in early 2023
Presented by: Declan Murphy, MB, BCH, BaO, FRACS, FRCS, Urol, Peter MacCallum Cancer Center, Melbourne, Australia
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Association of Urology (EAU) Annual Hybrid Meeting, Amsterdam, NL, Fri, July 1 – Mon, July 4, 2022.
References:
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021 Feb 27;397(10276):797-804.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.
Related Content: EAU 2022: Discussion: LuTectomy – A Single-arm Study of the Dosimetry, Safety, and Potential Benefit of 177Lu-PSMA-617 Prior to Prostatectomy


