(UroToday.com) The 37th Annual European Association of Urology Congress held in Amsterdam, Netherlands between July 1st,and 4th 2022 was host to an abstract session regarding improvements in metastatic prostate cancer with a focus on imaging and treatment.
Dr. Vjaters began his presentation by noting that darolutamide, a structurally distinct and highly potent androgen receptor inhibitor, has demonstrated a favorable safety profile in patients with non-metastatic castration-resistant prostate cancer (nmCRPC) per results from the phase 3 ARAMIS trial.1 The difference in the incidence of classic androgen receptor inhibitor adverse events between the darolutamide and placebo arms were <2%. Accordingly, the discontinuation rates due to adverse events remained consistently low and similar to placebo after longer follow-up periods (8.9% versus 8.7%). The recently reported ARASENS trial in metastatic hormone-sensitive patients similarly demonstrated non-significant incidences of adverse events in the darolutamide and placebo groups, with the highest incidences occurring during the overlapping docetaxel treatment periods.2
Three previous phase 1/2 studies (ARADES, ARAFOR, and a Japanese phase 1 trial) have demonstrated that darolutamide is well tolerated for up to 25 months in patients with mCRPC.3-5 Patients with mCRPC were pooled from these three phase 1/2 studies for this integrated analysis of long-term safety outcomes.
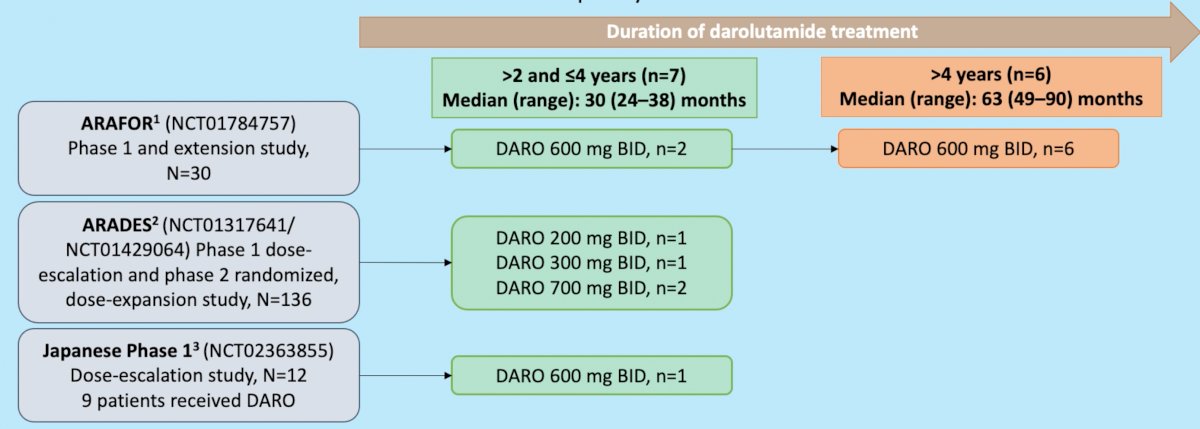
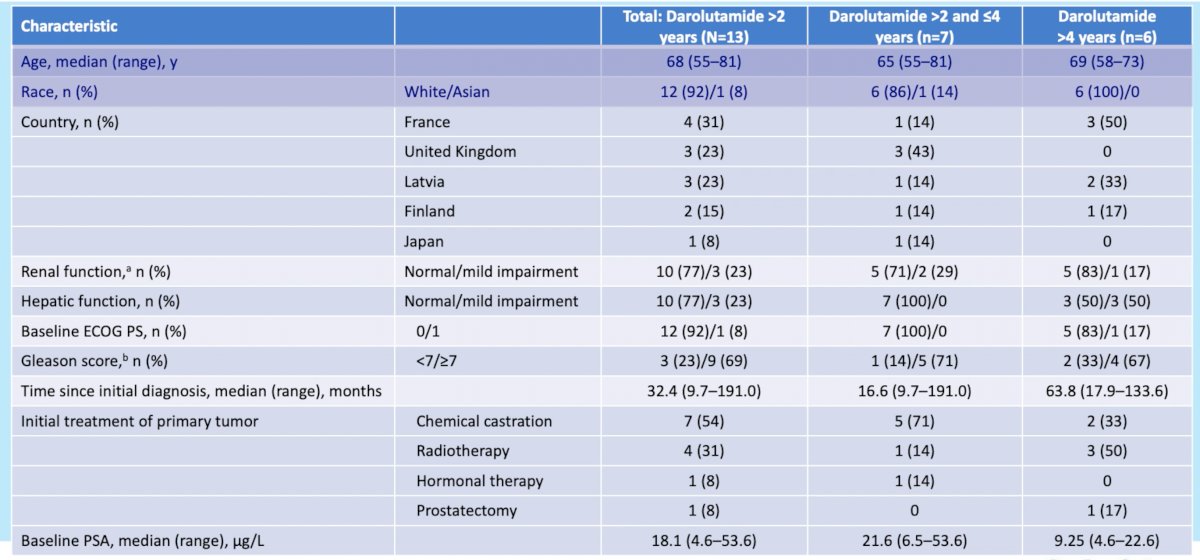
Baseline patient characteristics of the 13 patients with >2 years follow up included in this analysis are demonstrated in the table below:
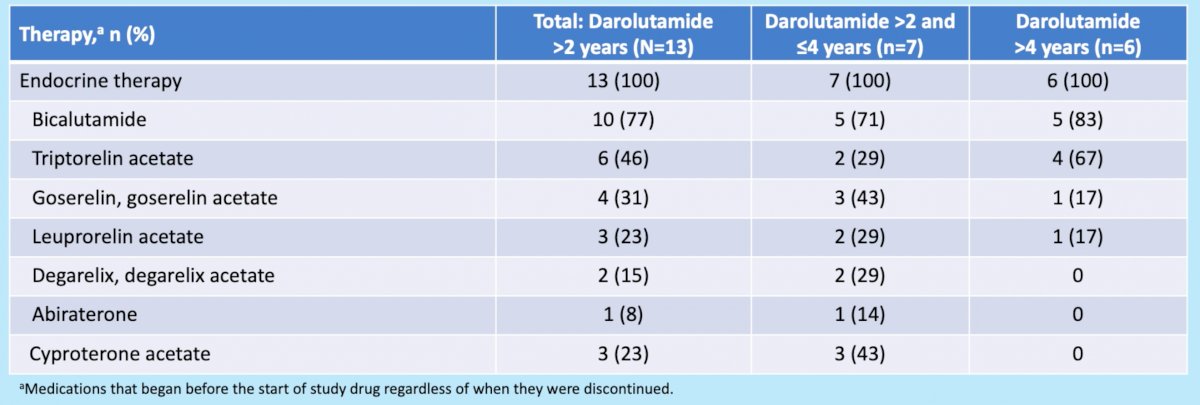
Notably, all patients received endocrine/hormonal therapy prior to darolutamide treatment:
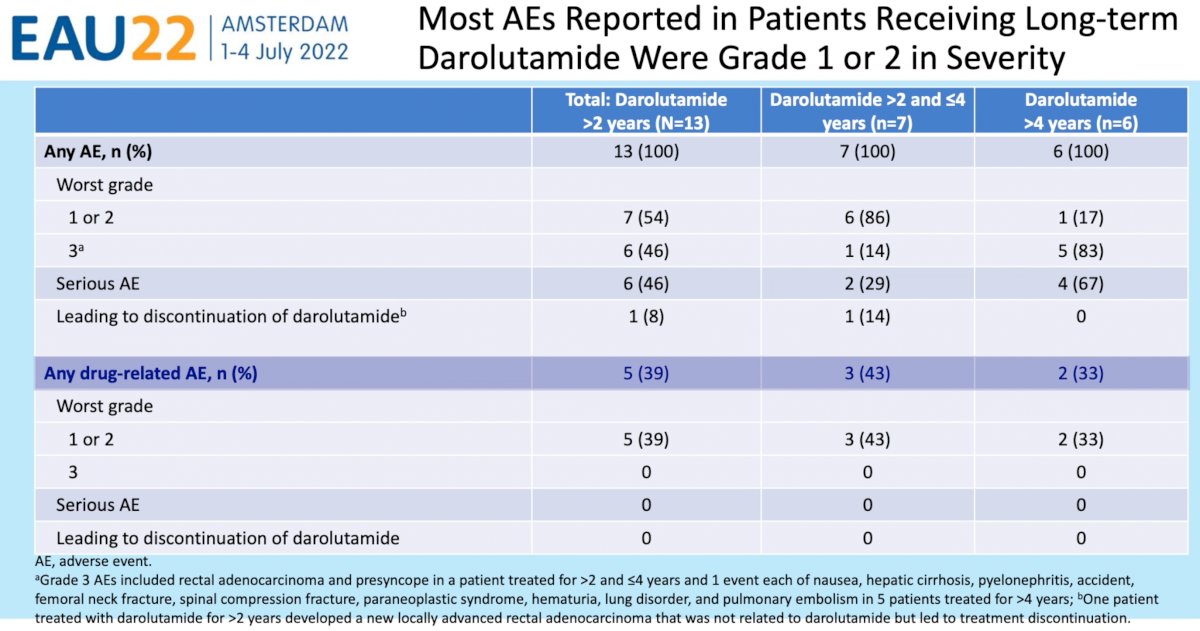
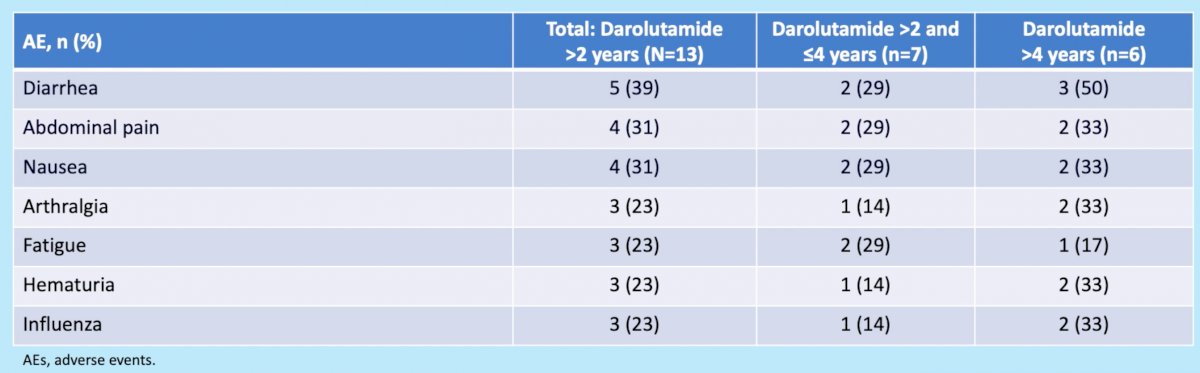
With regards to adverse events, all 13 patients reported an adverse event (seven grade 1 or 2 and six grade 3). Serious adverse events were reported in 6 (46%) of patients with 4 (67%) occurring in those on darolutamide for >4 years, with none of these adverse events attributed to darolutamide. No treatment discontinuations due to darolutamide-related adverse events were encountered. Five patients (39%) reported drug-related adverse events, none of which were grade 3 or worse.
The most common adverse events were diarrhea, abdominal pain, and nausea.
Dr. Vjaters concluded as follows:
- Long-term exposure to darolutamide in this small group of patients with mCRPC was well tolerated
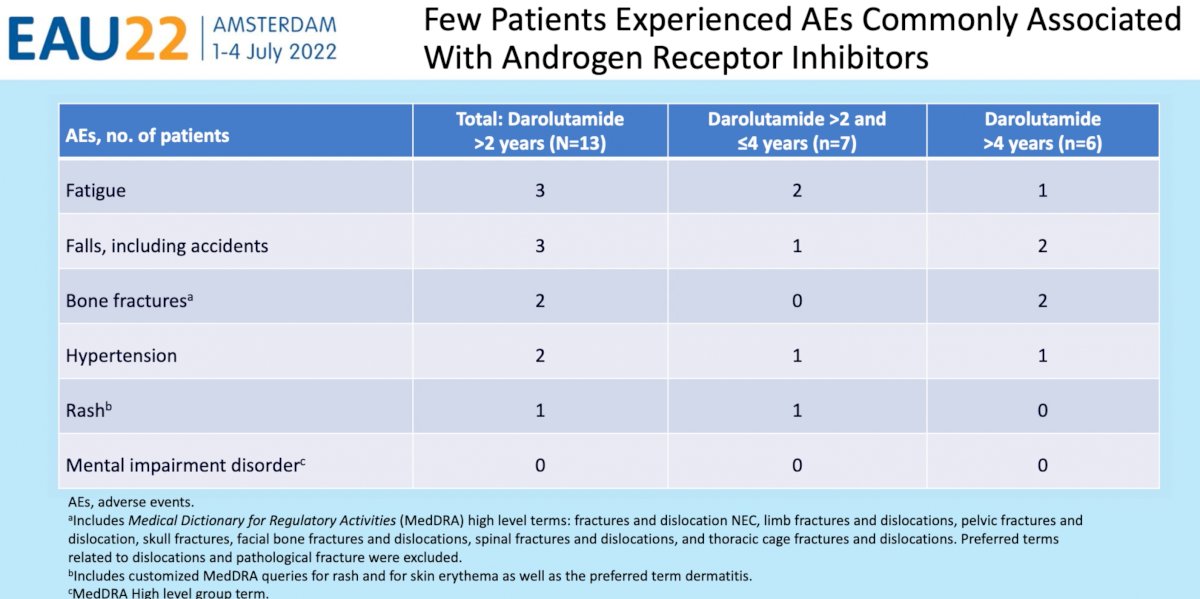
- Few patients developed adverse events commonly associated with androgen receptor inhibitors during long-term darolutamide treatment
- These findings are consistent with those previously reported, demonstrating a favorable safety and tolerability profile of darolutamide
Presented by: Dr. Egils Vjaters, MD, Professor, Department of Urology, P. Stradins Clinical University Hospital, Dept. of Urology, Riga, Latvia
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2022 European Association of Urology (EAU) Annual Hybrid Meeting, Amsterdam, NL, Fri, July 1 – Mon, July 4, 2022.
References:
- Fizazi K, et al. Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med. 2019;380:1235-46.
- Smith MR, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022;386(12):1132-42.
- Masard C, et al. Pharmacokinetics, Antitumor Activity, and Safety of ODM-201 in Patients with Chemotherapy-naive Metastatic Castration-resistant Prostate Cancer: An Open-label Phase 1 Study. Eur Urol. 2016;69(5):834-40.
- Fizazi K, et al. Activity and safety of ODM-201 in patients with progressive metastatic castration-resistant prostate cancer (ARADES): an open-label phase 1 dose-escalation and randomised phase 2 dose expansion trial. Lancet Oncol. 2014;15(9):975-85.
- Matsubara N, et al. Phase 1 study of darolutamide (ODM-201): a new-generation androgen receptor antagonist, in Japanese patients with metastatic castration-resistant prostate cancer. Cancer Chemother Pharmacol. 2017;80:1063-72.


