(UroToday.com) The 37th Annual European Association of Urology Congress held in Amsterdam, the Netherlands between July 1st, and 4th 2022 was host to an abstract session regarding improvements in metastatic prostate cancer with a focus on imaging and treatment. Dr. Crawford presented the results of his group’s study evaluating risk of major adverse cardiovascular events (MACE) following androgen deprivation therapy (ADT) initiation by cardiovascular (CV) history.
Dr. Crawford began his presentation by noting that the association between ADT use and increased CV risk in PCa patients is controversial. Analysis from the HERO trial by Shore et al. published in The New England Journal of Medicine in 2020 demonstrated that the risk of MACE was 2.9% in patients receiving relugolix (oral GnRH antagonist) compared to 6.2% in those receiving an LHRH agonist.1 However, a meta-analysis from 2011 showed no significant association between ADT exposure and risk of CV death.2 It is not known yet whether CV risk is conferred by ADT itself or other comorbidities. One potential risk factor for increased CV risk is a previous history of an CV event, as has previously been demonstrated.
The authors analysed data from a US electronic medical records database (DRG) that included 45,059 patients receiving LHRH agonist and antagonist injections between 2010 and 2020. MACE risk after ADT initiation was evaluated for patients both with and without a history of a MACE. The database contained 178,388 LHRH agonist and antagonist injection entries and 965 documented MACE events. MACE was defined as either a myocardial infarction, stroke, or death from any cause, consistent with previous studies. Patients were excluded if they experienced a MACE 6 months prior to start of ADT use. Kaplan-Meier survival curves were used to compare MACE rates in patients with and without MACE history with statistical comparisons conducted using the log-rank test.
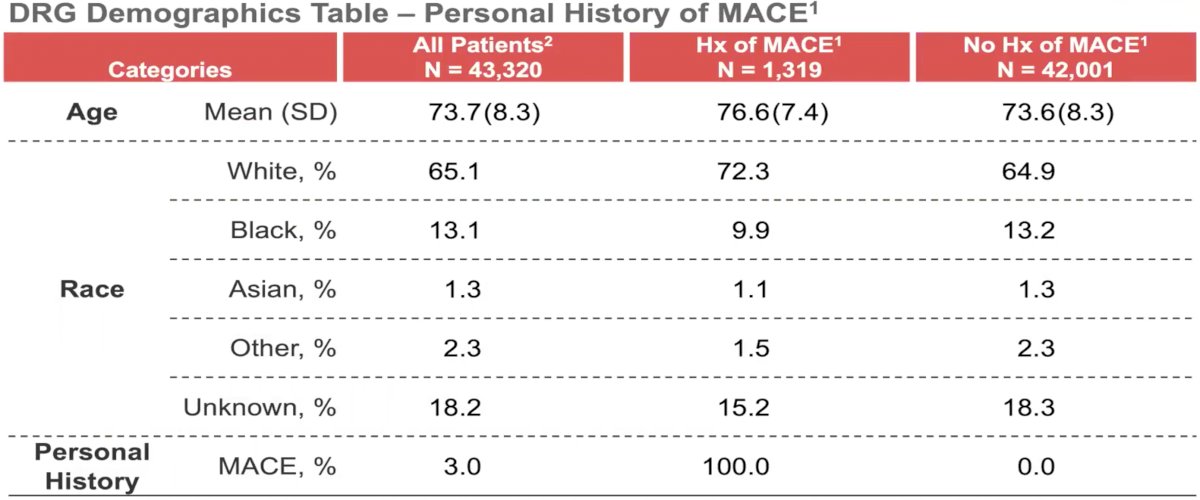
Baseline patient demographics are listed below:
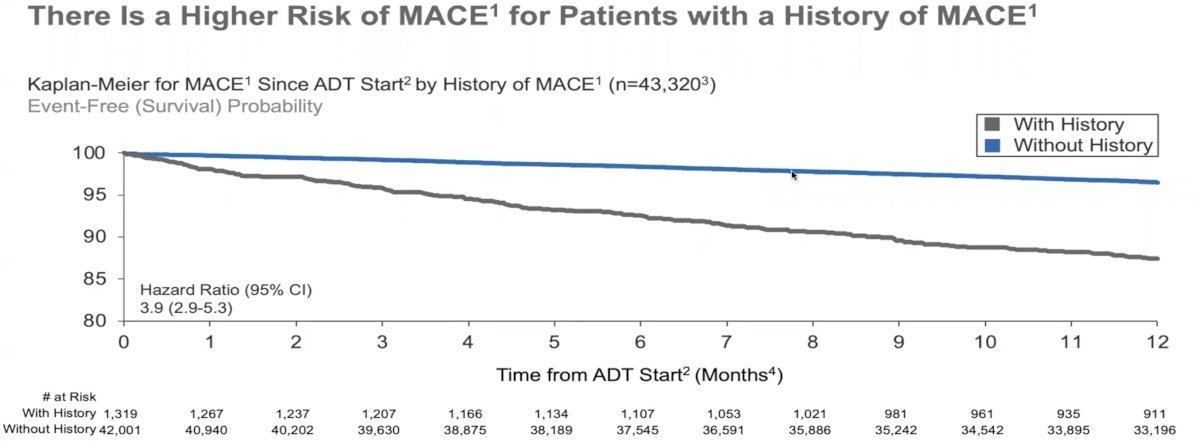
As expected, history of MACE prior to ADT start was associated with a 3.9-fold increased hazard of experiencing a further MACE event compared to those with no prior history of a MACE (HR: 3.9, 95% CI: 2.9 – 5.3).
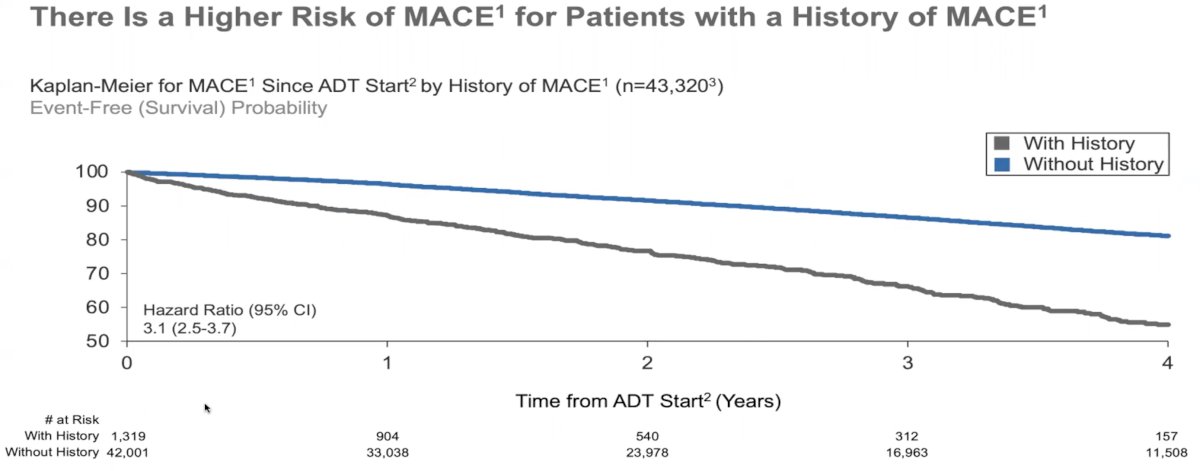
With extended follow up beyond 12 months, consistent results were seen (HR: 3.1, 95% CI: 2.5 – 3.7).
The authors concluded as follows:
- MACE risk following ADT initiation was higher for patients with a prior MACE history
- These findings are consistent with previous publications reporting elevated CV risk following CV events
- This analysis of data over 10 years from >44,000 PCa patients is likely an accurate reflection of the real world
Presented by: Dr. E. David Crawford, MD, Professor, Department of Urology, University of Colorado, Denver, CO
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2022 European Association of Urology (EAU) Annual Hybrid Meeting, Amsterdam, NL, Fri, July 1 – Mon, July 4, 2022.
References:
- Shore ND et al. Oral Relugolix for Androgen-Deprivation Therapy in Advanced Prostate Cancer. N Engl J Med. 2020;382(23):2187-96.
- Nguyen PL, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA. 2011;306(21):2359-66.


