(UroToday.com) The 37th Annual European Association of Urology Congress held in Amsterdam, the Netherlands between July 1st,and 4th 2022 was host to a session about the management of metastatic hormone sensitive prostate cancer (mHSPC). Dr. Eleni Efstathiou was tasked with discussing the current state of doublet versus triplet combination therapy in the management of metastatic hormone sensitive prostate cancer (mHSPC).
Dr. Efstathiou began by presenting the evolution of doublet therapy options (i.e. ADT + docetaxel or 2nd generation androgen receptor inhibitor [ARI]) in the mHSPC disease space from 2015 starting with the CHAARTED and STAMPEDE trials (ASCO 2015) to 2019 when TITAN and ENZAMET were presented at ASCO that year. Trials evaluating ADT + ARI have consistently demonstrated a survival benefit for doublet therapy over ADT alone (five out of five) compared to two out of three positive trials evaluating the ADT + docetaxel combination. This has further been supported by results from meta-analyses demonstrating the superiority of doublet therapy over ADT alone.
Furthermore, the early usage of doublet therapy with ARIs is critical. Early treatment with abiraterone in the LATITUDE trial conferred a 16.8 month overall survival benefit, compared to 4.6 and 4.4 months in the COU-AA-301 (mCRPC post-taxane) and COU-AA-302 (mCRPC pre-taxane) trials, albeit in the mCRPC space as opposed to the mHSPC with LATITUDE.
Although ARI doublet therapy has shown consistent survival benefits across the four different mHSPC subgroups (synchronous versus metachronous and high versus low volume metastatic disease burden), the same has not been demonstrated with docetaxel doublet therapy. Survival analysis from CHAARTED demonstrated that low volume mHSPC, particularly in the metachronous setting, does not derive benefit from Docetaxel intensification.1
As such, Dr. Efstathiou stated that by 2020, the following were the key points in the management of mHSPC patients:
- Doublet combination of ADT with either ARIs or Docetaxel is a requirement for the vast majority of mHSPC patients with appropriate monitoring.
- Addition of ARIs is likely the preferred choice over docetaxel for ‘garden variety’ mHSPC
- Combination should be initiated at diagnosis and not be delayed
At that time, the inevitable question was: is further treatment intensification better?
Results from the PEACE-1 trial were initially presented at ASCO 2021 with updated results presented at ESMO 2021 later in the year. The PEACE-1 trial employed a 2x2 design to assess, (separately and combined) the impact of the addition of abiraterone acetate/prednisone (AAP) and radiation (RT) to SOC therapy in men with mCSPC.
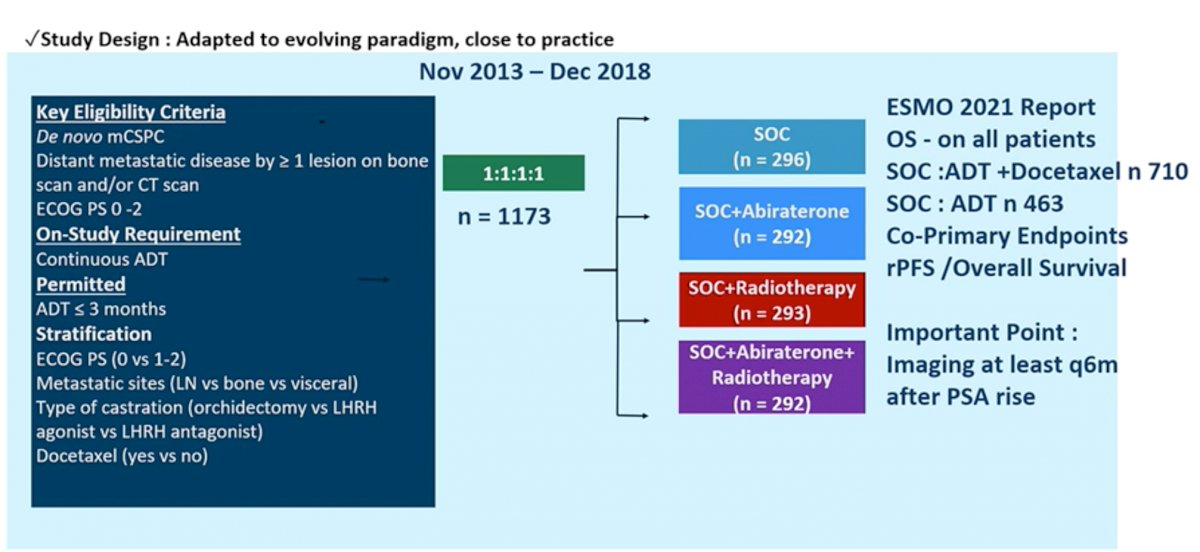
The control arm in this study evolved to reflect the contemporary SOC. When the study began enrolling patients, the SOC for first-line mCSPC was ADT alone. With publication of data from the STAMPEDE and CHAARTED studies, SOC was updated to include ADT plus Docetaxel 75 mg/m2 every 3 weeks for 6 cycles. The PEACE-1 study randomized 1,173 patients with 355 each to the SOC (with or without RT) and the SOC (with or without RT) plus AAP arm. Approximately two-thirds of patients on each arm had high-volume disease.
PEACE-1 was designed with two co-primary endpoints for the AAP analysis: radiographic progression-free survival (rPFS) and overall survival (OS). In the cohort of patients treated with SOC ADT plus Docetaxel (with or without RT), median follow-up was 3.8 years. The addition of AAP to SOC resulted in a 50% improvement in rPFS with median rPFS of 2.0 years on the SOC arm and 4.5 years on the SOC plus AAP arm (HR 0.50, 95% CI 0.40-0.62). rPFS benefit was greater in the high volume than low volume subgroups, but both populations benefited. Among patients with high volume disease, the addition of AAP to SOC resulted in a 53% improvement in rPFS with median rPFS of 1.6 years on the SOC arm and 4.1 years on the SOC plus AAP arm (HR 0.47, 95% CI 0.36-0.60). Likewise, the addition of AAP to SOC in patients with low volume disease resulted in a 42% improvement in rPFS with median rPFS of 2.7 years on the SOC arm versus not yet reached on the SOC plus AAP arm (HR 0.58, 95% CI 0.39-0.87).
Similar results were observed for OS. Starting with the overall study population (SOC of ADT with or without docetaxel), the addition of AAP resulted in an 18% improvement in OS with median OS of 4.7 years on the SOC arm and 5.7 years on the SOC plus AAP arm (HR 0.82, 95% CI 0.69-0.98). Limiting to the cohort of patients who received ADT plus Docetaxel as SOC, the addition of AAP resulted in a 25% improvement in OS with median OS of 4.4 years on the SOC arm versus not yet reached on the SOC plus AAP arm (HR 0.75, 95% CI 0.59-0.95). This effect was seen across subgroups, including those with high volume disease (HR 0.72, 95% CI 0.55-0.95) and low volume disease (HR 0.83, 95% CI 0.50-1.38); interaction P-value 0.64. Notably, the OS data is immature for the low volume patients due to a small number of events.

Again, we note improvement in OS with addition of ARIs, irrespective of docetaxel usage as SOC and metastatic disease burden.
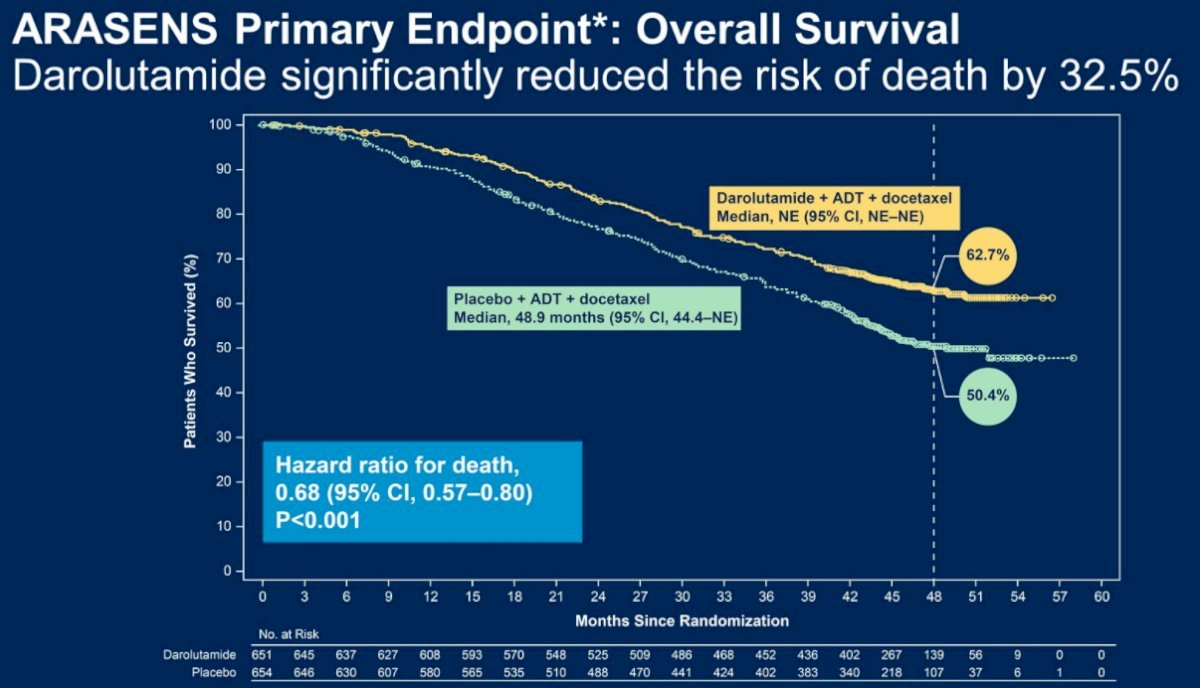
Likewise, results from ARASENS published in The New England Journal of Medicine in February 2022 have similarly demonstrated that the addition of Darolutamide to SOC ADT + Docetaxel in mHSPC patients is associated with significant improvements in 4-year overall survival from 50.4% to 62.7% (HR: 0.68, 95% CI: 0.57 – 0.80, p<0.001).2
Dr. Efstathiou concluded with these key principles for treatment of fit patients with mHSPC in 2022:
- Monotherapy with ADT is unethical
- Doublets
- ADT + ARIs is a requirement
- ADT + Docetaxel alone is not an acceptable choice
- Triplets: ADT + Docetaxel + ARIs is a choice (acceptable especially for de novo metastatic high-volume disease)
Presented by: Dr. Eleni Efstathiou, MD, PhD, Associate Professor, Genitourinary Oncology Section Chief, Houston Methodist Cancer Center, Houston, TX
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2022 European Association of Urology (EAU) Annual Hybrid Meeting, Amsterdam, NL, Fri, July 1 – Mon, July 4, 2022.
References:
- Kyriakopoulos CE, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J Clin Oncol. 2018;36(11):1080-7.
- Smith MR, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. New Engl J Med. 2022;386(12):1132-42.


