(UroToday.com) The 37th Annual European Association of Urology Congress held in Amsterdam, Netherlands between July 1st, and 4th 2022 was host to a session addressing controversies in urologic oncology. Dr. Christopher Sweeney was tasked with arguing in favor of systemic therapy in prostate cancer patients with PET-detected oligorecurrent prostate cancer.
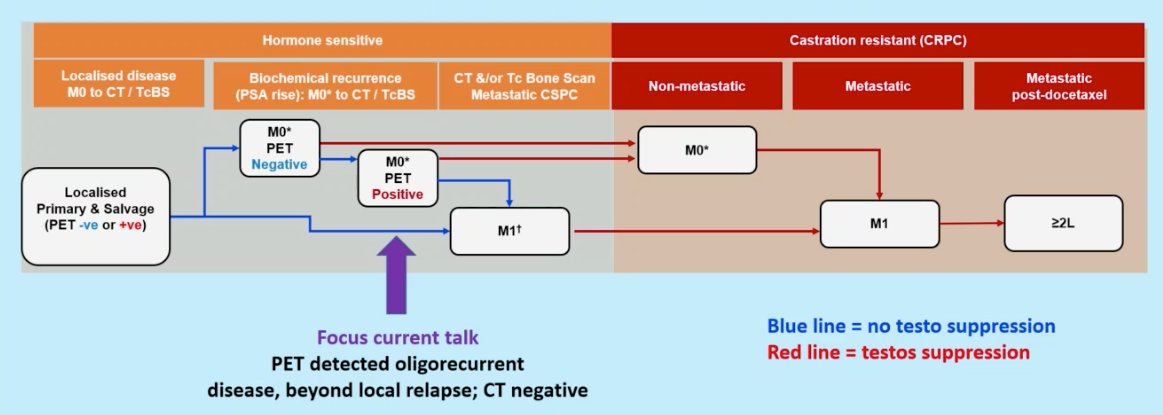
Dr. Sweeney began by defining the cohort of PET-detected oligorecurrent prostate cancer. Typically, this is a cohort of patients that experiences a rising PSA following definitive local therapy who subsequently are found to have no evidence of metastasis on conventional imaging, but with continued rising PSA levels, are found to have low volume metastasis on PET imaging.
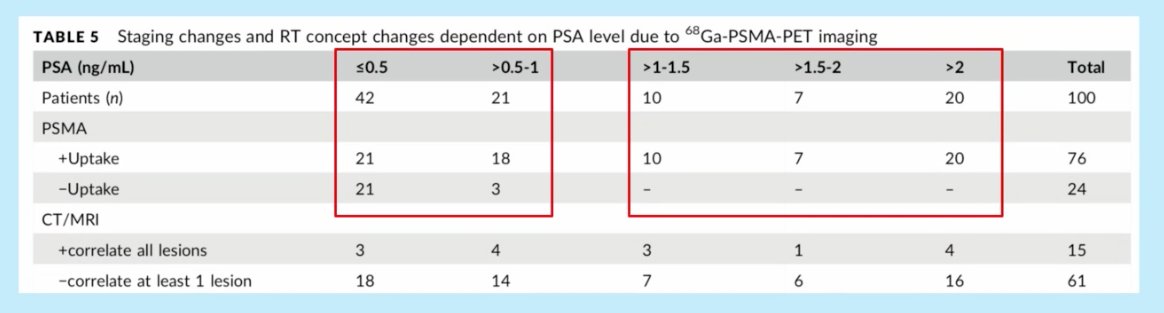
The higher the PSA level, the higher the odds of having a positive PSMA PET scan. Habl et al. demonstrated that for patients with PSA <0.5 ng/ml, 50% of patients have a positive PSMA scan. Conversely, 100% of patients with PSA >1 ng/ml have evidence of uptake on their PSMA scans.1
But should we rush to treat patients with rising PSAs post-local therapy, who may or may not have evidence of oligometastatic disease on PET scans if performed?
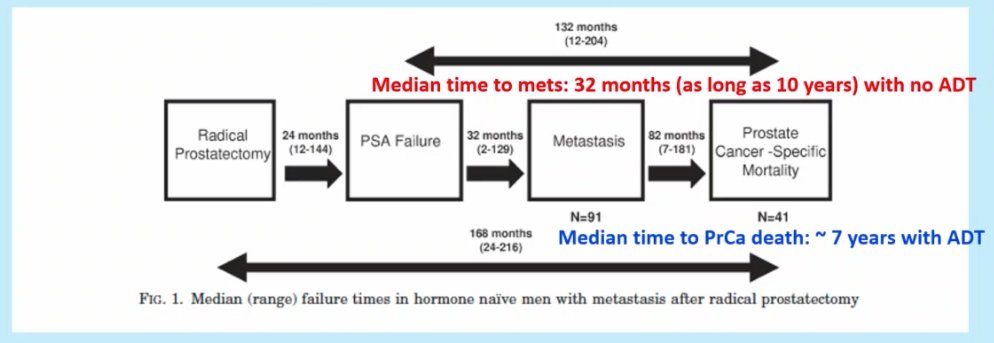
Dr. Sweeney referenced data from the Johns Hopkins University post-prostatectomy cohort that evaluated the natural history of such patients. Of 3,096 patients who underwent a radical prostatectomy, 422 (13.6%) had a PSA rise >0.2 ng/dL with these patients surveilled until the development of metastasis (no ADT). 91 patients (21.2% of those with BCR) developed metastatic disease and 41 patients died of prostate cancer (more than half with non-prostate cancer deaths). Of most significance though is the long natural history of this disease process. The median time from PSA failure to metastasis was 32 months (and as long as 10 years) with no ADT. As such Dr. Sweeney has adopted this approach in a number of patients in his practice. The median time from metastasis to prostate cancer death was ~7 years with ADT,2 which is likely to be longer in 2022 given the advances in the treatment of advanced prostate cancer patients since the study period. Again, the importance of considering a patient’s age/performance status balanced with the competing risk of non-prostate cancer-related mortality is critical.
What about the evidence for metastasis-directed therapy (MDT) in patients with oligometastatic disease following treatment with curative intent?
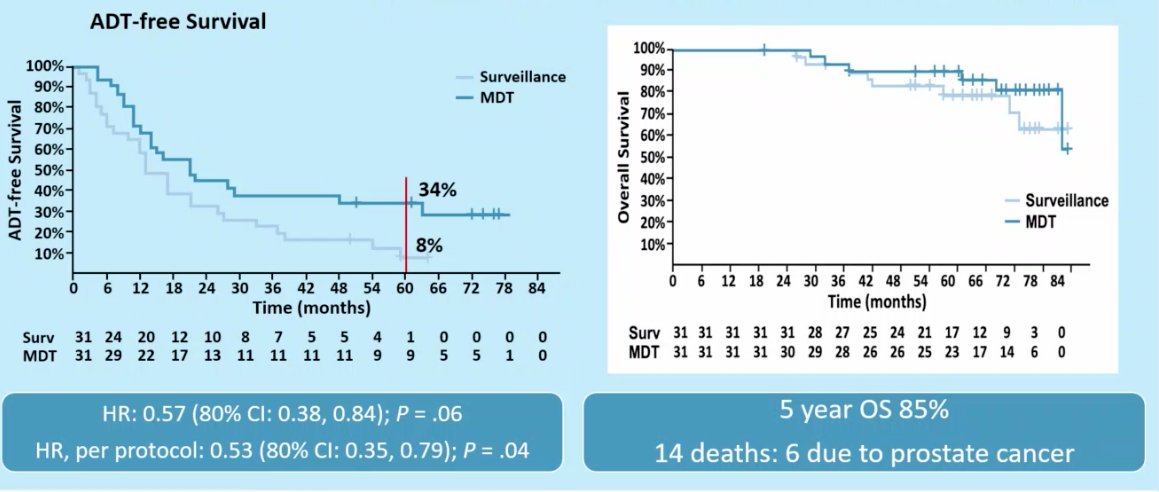
STOMP included patients with asymptomatic prostate cancer with biochemical recurrence following treatment with curative intent. Patients were eligible if testosterone was >50 ng/mL and a choline PET-CT revealed as many as 3 extracranial sites of disease. Patients were randomized 1:1 and MDT was either stereotactic body radiotherapy (SBRT) or metastasectomy. The primary endpoint was time to initiation of ADT (ADT-free survival). ADT was initiated for symptoms, progression beyond 3 metastases, or local progression of known metastatic disease.
This trial demonstrated that MDT improved ADT-free survival at five years from 8% to 34% (HR: 0.57, 95% CI :0.38-0.84, p=0.06). This did not translate to improved OS rates (85% at 5 years in both arms). Notably, of the 14 patients who died in this cohort, 8 (57.1%) died from non-prostate cancer related causes.
The 85% 5-year OS is similar to that seen with trials comparing intermittent and continuous ADT, which demonstrated 5-year OS rates of ~80% with a median OS of 8 years in patients with rising PSA post-RP/XRT and negative CT A/P and bone scans (possibly PSMA-PET +ve?). Again, 60% of the 524 deaths in this cohort were not prostate cancer related.
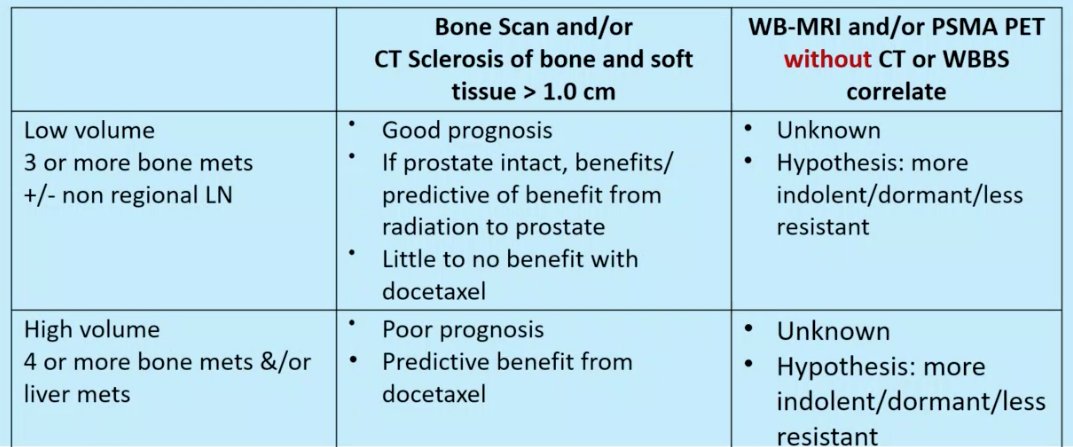
Not all bone metastases are created equally. Dr. Sweeney went on to discuss the differences between CT/bone scan detected lesions and PSMA-PET/MRI detected lesions.
- For CT or bone scans to detect lesions, the disease bulk needs to be larger with lesions >1 cm needed in LNs or body organs.
- Is this associated with more clones and thus more resistance?
- These lesions are also likely to cause changes to bone
- The biology enables the lesions to get a foothold in the bone microenvironment, making these lesions more resistant/adaptable/aggressive
Chance of eradication with intense systemic therapy becomes very low
- For PSMA PET/MRI detected lesions without CT or bone scan findings
- Evaluates cancer directly (bone scan detects bone changes not bone lesions specifically)
- Smaller disease bulk and thus less clones with less resistance
- Does not get a foothold in bone and thus less resistant to therapy as not protected by the bone microenvironment
Possibly eradicate with intense systemic therapy in the adjuvant setting
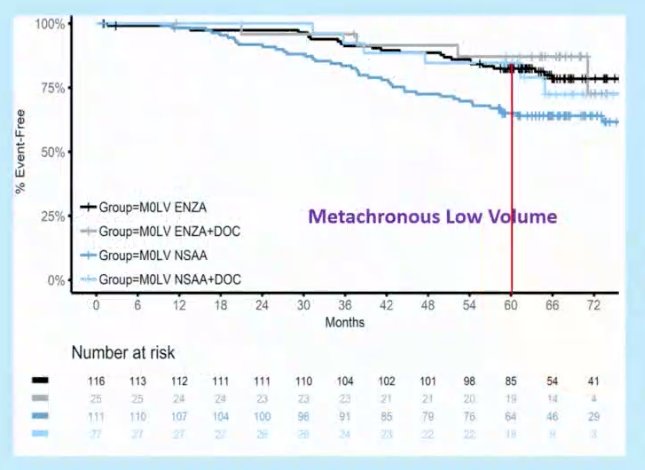
Dr. Sweeney next proposed a framework to understand different information from bone scan/CT versus PSMA-PET/MRI without CT correlates. He highlighted that recent data has shown that docetaxel has no survival benefit when added to ADT in patients with metachronous, low volume mHSPC on conventional imaging, and thus has no role currently in the management of the oligometastatic patients diagnosed on PSMA PET/CT.
There is currently a lack of clear guidance about the most appropriate management for such patients. No documented life-prolonging therapies for nmHSPC exist.
- These patients have variable treatment courses
- Treatment and patient selection remain unclear due to lack of randomized data
- SBRT for PSMA PET-positive lesions remains an option
- Intermittent hormone therapy is preferred if used
- Death due to other causes during this disease state is not uncommon
It is important to understand new and emerging data. Clinicians must work together to develop treatment consensus. Clinicians must engage patients and consider their preferences. Management must be individualized, and treatment depends on prognostic features, quality of life effects, duration of therapy, and patient preferences.
Based on a survey of attendees from the APCCC asking about timing of long-term ADT in patients with non-metastatic disease and confirmed rising PSA (post-local therapy plus/minus salvage local radiotherapy), 80% of attendees responded that they would start long-term ADT only in a minority of selected high-risk patients, e.g. PSA ≥4 ng/ml and rising, with PSA doubling time <6 months OR PSA ≥20 (STAMPEDE criteria).
In contrast to docetaxel in patients with low volume, metachronous mHSPC, enzalutamide addition to standard ADT appears to confer a survival benefit based on data from ENZAMET presented at ASCO 2022. Enzlutamide addition to ADT in these patients improved 5-year OS from 65% to 85%. Thus, one wonders if a similar survival advantage could be seen with taking this approach one step “back” for PSMA-detected oligorecurrent disease occult to conventional scans.
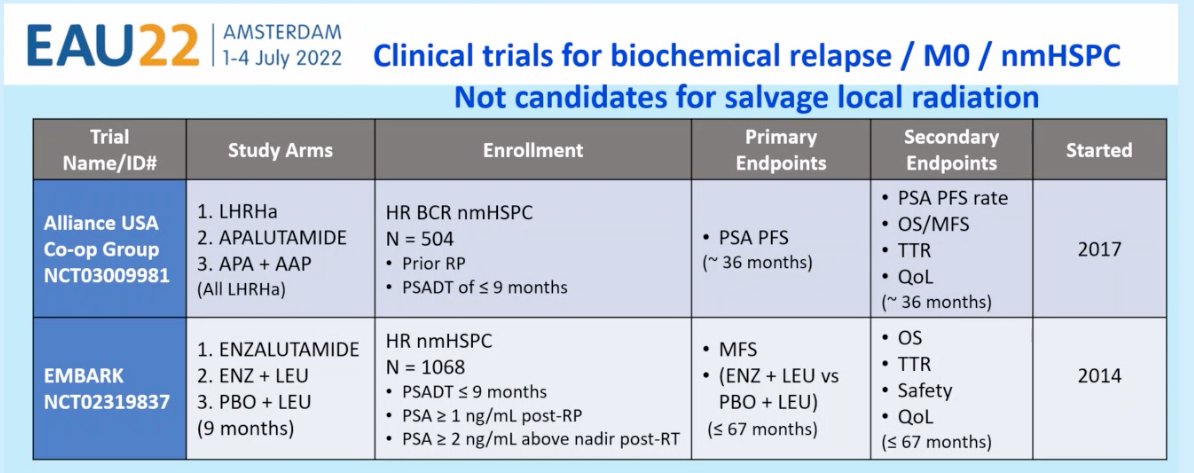
Current trials in the biochemical recurrent, M0, nmHSPC disease include the:
- Alliance USA Co-op Group trial (NCT03009981): A randomized, open-label, three arm phase 3 study of a) degarelix monotherapy compared to each of two experimental arms: b) degarelix plus apalutamide, and c) degarelix plus apalutamide plus abiraterone acetate/prednisone, in pts with biochemical recurrence following prior RP without metastases on conventional imaging, and a PSADT of ≤ 9 months.
- EMBARK trial: a randomised, phase 3 study of high-risk patients with nmHSPC, a PSADT of ≤9 months and a screening PSA of ≥2 ng/mL above the nadir after radiotherapy (RT) or ≥1 ng/mL after radical prostatectomy (RP) with or without postoperative RT. Men (n=1050) are randomised 1:1:1 to enzalutamide 160 mg/day plus LHRHa or placebo plus LHRHa (double-blind arms) or enzalutamide monotherapy (open-label arm).
Dr. Sweeney ended his presentation with the following “scorecard” for treatment options for PET-detected, CT/bone scan negative oligorecurrent disease, emphasizing the importance of informing patients of all the risks and benefits and shared decision making.

Presented by: Christopher Sweeney, MBBS, Professor, Medical Oncology, Department of Medicine, Dana-Farber Cancer Institute, Boston, MA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2022 European Association of Urology (EAU) Annual Hybrid Meeting, Amsterdam, NL, Fri, July 1 – Mon, July 4, 2022.
References:
- Habl G, et al. 68Ga-PSMA-PET for radiation treatment planning in prostate cancer recurrences after surgery: individualized medicine or new standard in salvage treatment. Prostate. 2017;77(8):920–7.
- Makarov DV, et al. The natural history of men treated with deferred androgen deprivation therapy in whom metastatic prostate cancer developed following radical prostatectomy. J Urol. 2008;179(1):156-61.
Related Content: EAU 2022: How Should PET-Detected Oligorecurrent Prostate Cancer Best Treated? Metastasis-Directed Therapy


