(UroToday.com) The 2022 EAU annual meeting featured a game changing session, including a discussant presentation by Dr. Juan Gomez Rivas discussing exploratory endpoints from PROpel, a phase III trial of abiraterone + olaparib versus abiraterone + placebo in first-line mCRPC. Dr. Gomez Rivas notes that there is an evolving treatment paradigm for prostate cancer. Among patients diagnosed with localized prostate cancer, 90% of those patients are treated with radiotherapy, surgery or active surveillance, leading to a cure in ~75% of those patients, with the remaining 25% being treated with androgen deprivation therapy (ADT) in the setting of biochemical recurrence. Among these patients, a proportion will develop M0 castration resistant prostate cancer (CRPC), for which the SPARTAN trial,1 PROSPER trial,2 and ARAMIS trial3 demonstrated a survival benefit vs placebo for apalutamide, enzalutamide, and darolutamide, respectively. For patients that have metastatic castration sensitive prostate cancer (mCSPC), docetaxel (CHAARTED,4 STAMPEDE5), abiraterone (LATITUDE,6 STAMPEDE7), enzalutamide (ARCHES,8 ENZAMET9), and apalutamide (TITAN10) have all improved survival. However, patients may progress to require additional treatments in the M1 CRPC setting, including several additional lines of therapy:

Dr. Gomez Rivas notes that there are several clinical factors that support treatment choice. For those considering hormone therapy:
- Less severe toxicity
- Asymptomatic patients
- Frail/elderly patients
- Oral administration
For those considering chemotherapy:
- Known toxicity profile
- Symptomatic patients
- “Fit” for chemotherapy
- “Aggressive” tumors
For those considering Radium-223:
- Favorable safety profile
- Bone-dominant disease
- Symptomatic patients
- After chemotherapy
Sequence of therapy, considered the “holy grail” in treatment of advanced prostate cancer, is also important as demonstrated by the PSA-RR, PFS, and OS for the following situations of abiraterone after enzalutamide +/- docetaxel, enzalutamide after abiraterone +/- docetaxel, and cabazitaxel after docetaxel and abiraterone/enzalutamide:
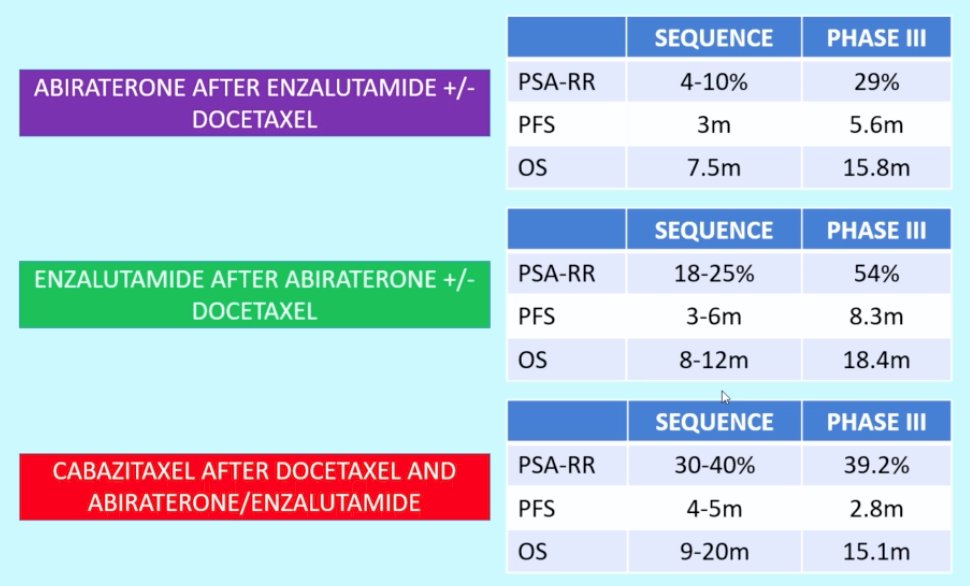
Importantly, there is a need for precision-medicine strategies in a prostate cancer. For those with indolent prostate cancer, who does not need active therapy? For those with curable prostate cancer, who suffers relapse? Who should get surgery versus radiation? Who would benefit from more aggressive therapeutic approaches? For those with lethal prostate cancer, what is the optimal sequential use of standard therapies for each patient? Who should receive more intensive therapy upfront? How do we personalize therapy based on targetable alterations (ie. DDR, PI3K)?
Dr. Gomez Rivas then discussed PROpel, a global, randomized, double-blind phase III trial randomizing men with mCRPC to first-line abiraterone + olaparib vs abiraterone + placebo in a 1:1 fashion. The primary endpoint is rPFS by investigator assessment and by blinded independent review, whereas the key secondary endpoint is overall survival. Additional key endpoints include: (i) time to first subsequent therapy or death (TFST), (ii) time to second progression or death (PFS2), (iii) objective response rate (ORR), (iv) HRR gene mutation status (by tissue and ctDNA testing), (v) health-related quality of life, and (vi) safety and tolerability:

Dr. Gomez Rivas notes that there are several ways to measure clinical benefit in the advanced prostate cancer setting:
- Keeping the patient asymptomatic for as long as possible
- Delaying the onset of new metastasis
- Increasing patient survival
- Not limiting the usefulness of the following therapy lines and not creating cross-resistance or long-term side effects
In PROpel, the median rPFS for abiraterone + olaparib was 24.8 months vs 16.6 months for abiraterone + placebo (HR 0.66, 95% CI 0.54-0.81), demonstrating a 34% reduction in the risk of radiological progression or death with abiraterone + olaparib:
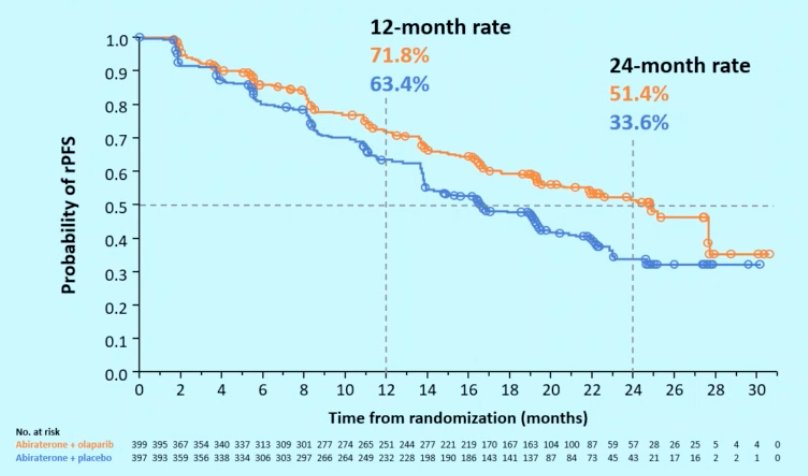
Can rPFS translate clinical benefit? Dr. Gomez Rivas notes that in other tumor sites (ie. breast, colorectal, gastric, non-small cell lung, ovarian, and kidney cancer) there have been studies suggesting correlation (strong relationship: correlation coefficient >= 0.7) between progression free survival and overall. Of note, we do not have these studies in prostate cancer, but these other solid tumor analyses provide some guidance. Dr. Gomez Rivas suggests that we may see a similar benefit in PROpel.
In PROpel, overall survival (28.6% maturity) showed a trend towards improvement with abiraterone + olaparib (HR 0.86, 95% CI 0.66-1.12), in addition to TFST (HR 0.74, 95% CI 0.61-0.90) and PFS2 (HR 0.69, 95% CI 0.51-0.94) results supporting longer-term benefit with abiraterone + olaparib:

Can exploratory endpoints translate into clinical benefit (ie. PSA kinetics)? In the M0CRPC setting we have seen an association of PSA decline with metastasis-free survival, symptomatic progression, and PFS2. In PROpel, there was a 10% higher confirmed PSA response rate with abiraterone + olaparib, and the median time to PSA response (abiraterone + olaparib not reached vs 12.0 months olaparib + placebo) favored the abiraterone + olaparib arm (HR 0.55, 95% CI 0.45-0.68):
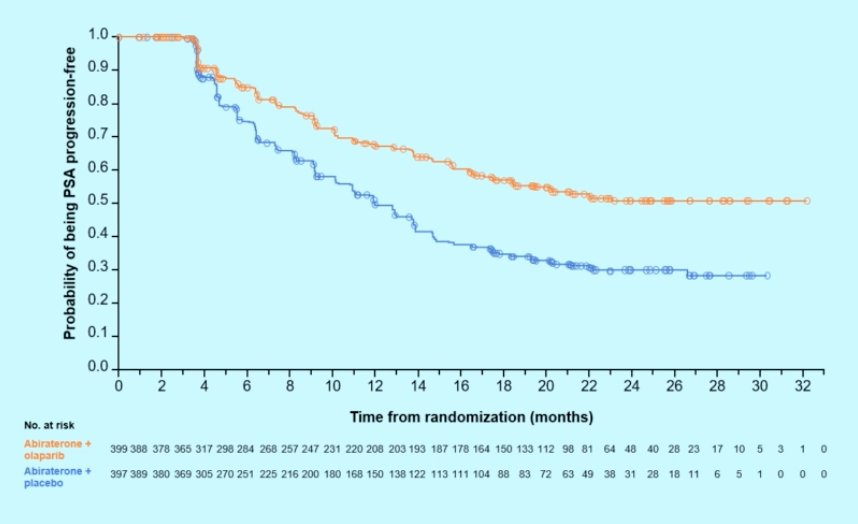
To conclude his discussant presentation, Dr. Gomez Rivas notes that looking at the four criteria for clinical benefit, PROpel provides the following benefits:
- Keeping the patient asymptomatic for as long as possible – not currently
- Delaying the onset of new metastasis – yes
- Increasing patient survival – not currently
- Not limiting the usefulness of the following therapy lines and not creating cross-resistance or long-term side effects – yes
Presented by: Juan Gomez Rivas, Department of Urology, Hospital Clinico San Carlos. Madrid, Spain.
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Association of Urology (EAU) Annual Hybrid Meeting, Amsterdam, NL, Fri, July 1 – Mon, July 4, 2022.
References:
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide and Overall Survival in Prostate Cancer. Eur Urol. 2021 Jan;79(1):150-158.
- Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med. 2020 Jun 4;382(23):2197-2206.
- Fizazi K, Shore N, Tammela TL, et al. Nonmetastatic, Castration-Resistant Prostate Cancer and Survival with Darolutamide. N Engl J Med. 2020 Sep 10;383(11):1040-1049.
- Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: Long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol 2018 Apr 10;36(11):1080-1087.
- Clarke NW, Ali A, Ingleby FC, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results in the STAMPEDE trial. Ann Oncol 2019 Dec 1;30(12):1992-2003.
- Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): Final overall survival analysis of a randomized, double-blind, phase 3 trial. Lancet Oncol 2019 May;20(5):686-700.
- James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med. 2017;377(4):338-351.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy with Enzalutamide or Placebo in Men with Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol. 2019 Nov 10;37(32):2974-2986.
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med 2019 Jul 11;381(2):121-131.
- Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: Final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. 2021 Jul 10;39(20):2294-2303.


