(UroToday.com) The 37th Annual European Association of Urology Congress held in Amsterdam, the Netherlands between July 1st, and 4th 2022 was host to an abstract session regarding new insights into the management of upper tract urothelial cancer (UTUC). Dr. Daichi Tamura presented his group’s work evaluating the utility of plasma and urinary circulating tumor DNA (ctDNA) in the detection of individualized mutations in patients with UTUC.
Dr. Tamura began his presentation by highlighting that UTUC is an aggressive disease with high recurrence and progression rates. Current standards for the diagnosis/detection of recurrences are either highly invasive or have low sensitivities. ctDNA, which can be performed via next generation sequencing (NGS) or digital polymerase chain reaction (dPCR), has shown promising results for monitoring several types of cancer.1
The aim of this study was to evaluate the validity of plasma and urinary ctDNA as UTUC tumor biomarkers. To that end, the authors included 23 patients who underwent a radical nephroureterectomy (RNU) for UTUC between January 2019 and December 2020. For each study patient, whole exome sequencing (WES) of both tumor DNA and corresponding peripheral blood mononuclear cells (PBMC) DNA was performed by NGS. The tumor-specific mutations were identified by comparing the single nucleotide variants from tumors and PBMCs. Primers and probes for each mutation were designed to investigate ctDNA by dPCR analysis. Plasma and urine ctDNA were obtained from each patient. Longitudinal variant allele frequency (VAF) of ctDNA was plotted in the perioperative period.

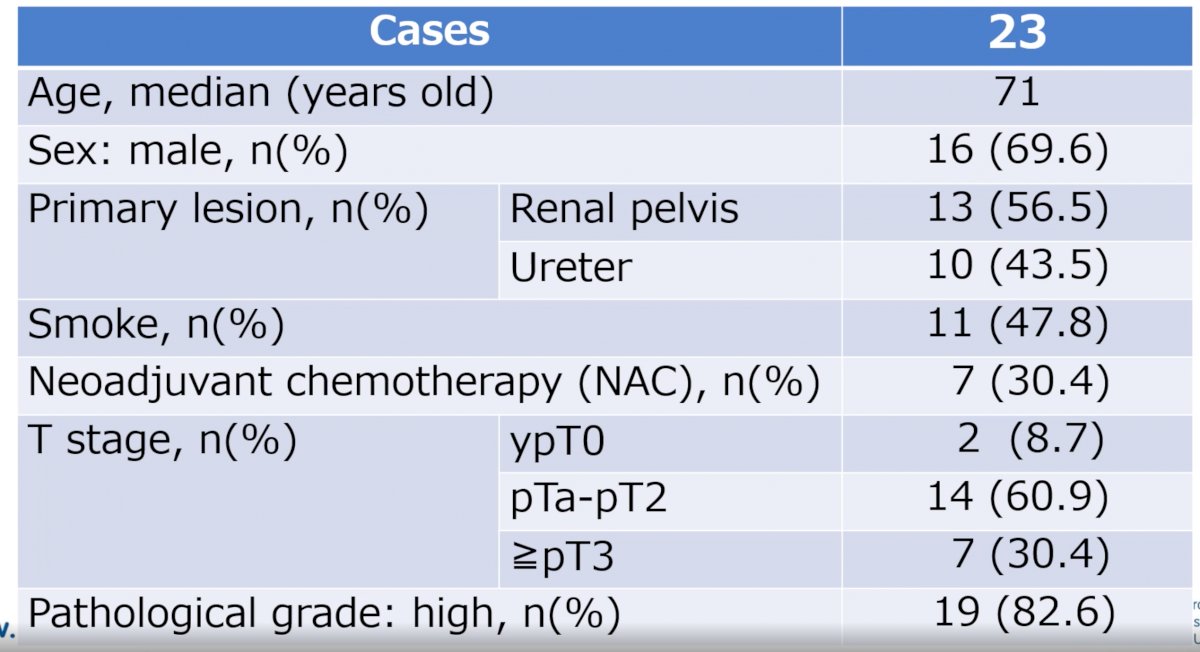
The median patient age was 71 years old (range: 57-82), 70% were male, 56.5% of tumor originated from the renal pelvis, 30.4% received neoadjuvant chemotherapy, and 82.6% had high grade disease (see table below):
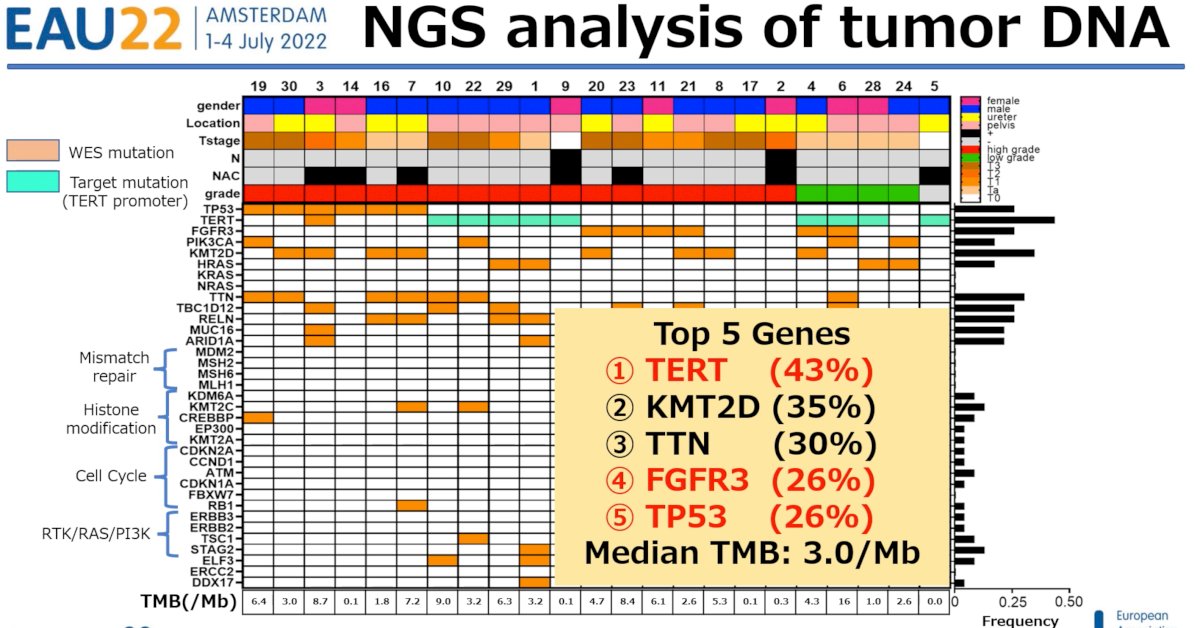
NGS analysis of tumor DNA demonstrated a median tumor mutational burden (TMB) of 3.02/Mb. The top five genes identified were:
- TERT
- KMT2D
- TTN
- FGFR3
- TP53
Dr. Tamura next presented results of plasma and urinary ctDNA monitoring using dPCR in 5 patients with detectable pre-operative tumor-unique mutations, who all subsequently had sustained decreases in the VAFs after RNU and, significantly, had no evidence of clinical recurrence (CT, cystoscopy, urinary cytology).
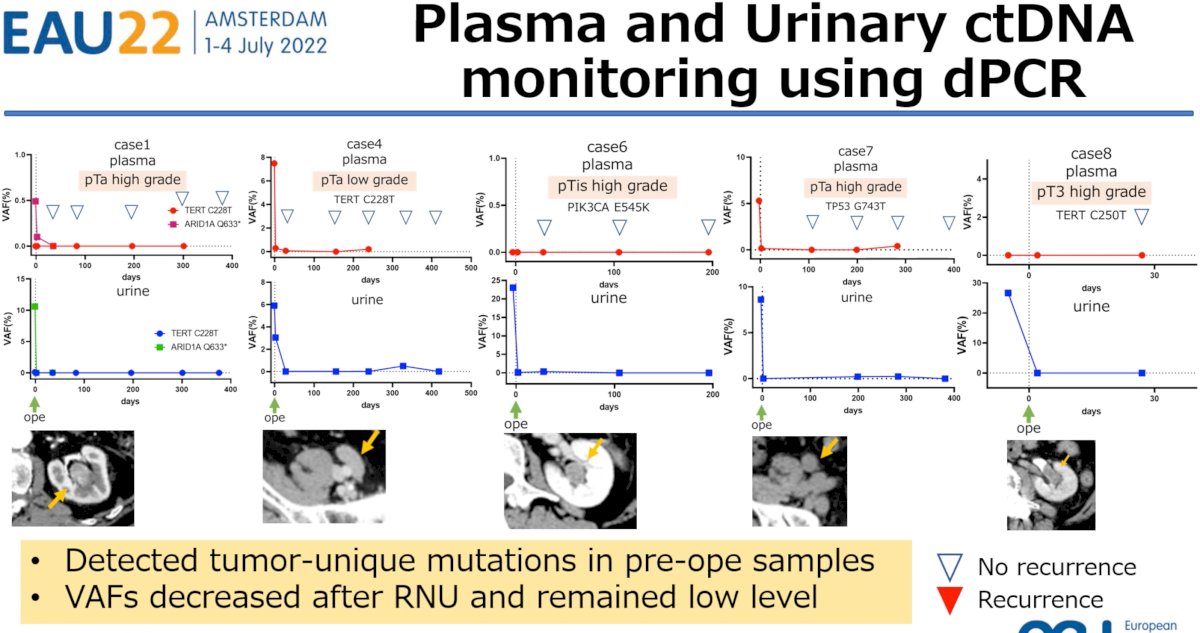
Conversely, 2 patients were noted to be ypT0 after receipt of neoadjuvant chemotherapy prior to RNU and subsequently developed clinical recurrence. Both patients had undetectable plasma and urinary ctDNA pre-operatively with subsequent increases during follow up. Of utmost clinical importance is the fact that urinary ctDNA increases were detected earlier than clinical recurrence.
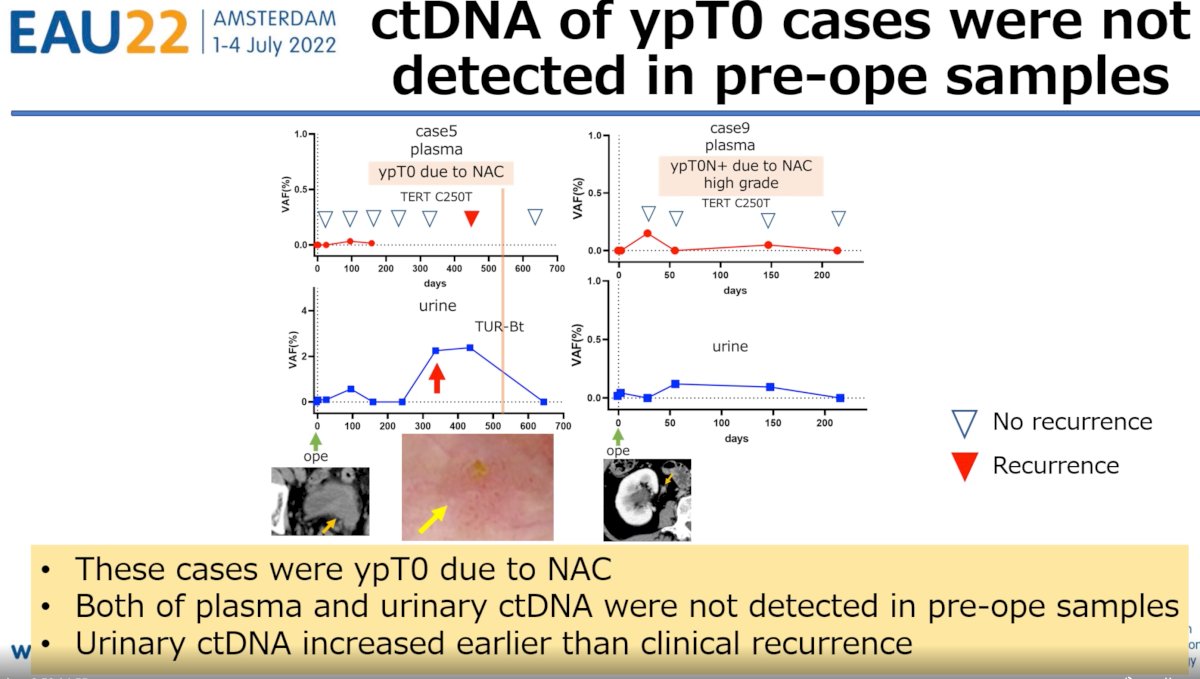
Dr. Tamura concluded his presentation with the following discussion points:
- High mutation frequency genes and TMB were similar to those reported in previous studies2
- The study group’s original primer-probe library including TERT, FGR3, and TP53 could cover most of UTUC during monitoring
- This study showed changes in VAFs of case-specific mutations using plasma and urine samples before and after RNU VAFs of ctDNA could be related to tumor burden
- Plasma and urinary ctDNA are useful biomarkers of UTUC
Presented by: Daichi Tamura, MD, Department of Urology, Iwate Medical University, Morioka, Iwate, Japan
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2022 European Association of Urology (EAU) Annual Hybrid Meeting, Amsterdam, NL, Fri, July 1 – Mon, July 4, 2022.
References:
- Corcoran RB, Chabner BA. Application of Cell-free DNA Analysis to Cancer Treatment. N Engl J Med. 2018;379(18):1754-65.
- Fujii Y, Sato Y, Suzuki H, et al. Molecular classification and diagnostics of upper urinary tract urothelial carcinoma. Cancer Cell. 2021;39(6):793-809.


