(UroToday.com) The 2023 European Association of Urology (EAU) annual congress held in Milan, Italy between March 10th and 13th, 2023 was host to a prostate cancer biopsy indication session evaluating the additive value of positron emission tomography (PET), micro-ultrasound, and/or markers in this setting. Dr. Philipp Krausewitz presented the study protocol and interim analysis for the Detection rate of clinically significant PROstate cancer by mpMRI and PSMA-PET/CT fusion biopsy (DEPROMP) trial.
Dr. Krausewitz began by highlighting that PSMA-PET/CT, in the primary staging setting, is associated with an improved detection of occult metastases, may improve local staging when combined with MRI, increases the sensitivity and negative predictive value for clinically significant prostate cancer (csPCa) on biopsy after MRI triage, and, currently, is estimated to influence treatment plans in 25 to 75% of cases. As such, the investigators hypothesized that PSMA-PET/CT may have additive value in the biopsy naïve setting.
The study design is as below. In brief, this trial will include 230 biopsy-naïve men with a high suspicion for prostate cancer (PSA >4 ng/ml, suspicious DRE or ultrasound), who will undergo an MRI + a PSMA-PET/CT, followed by a fusion biopsy. The primary outcome is the additive value of PSMA-PET/CT, when combined with MRI, in influencing medical decision making.
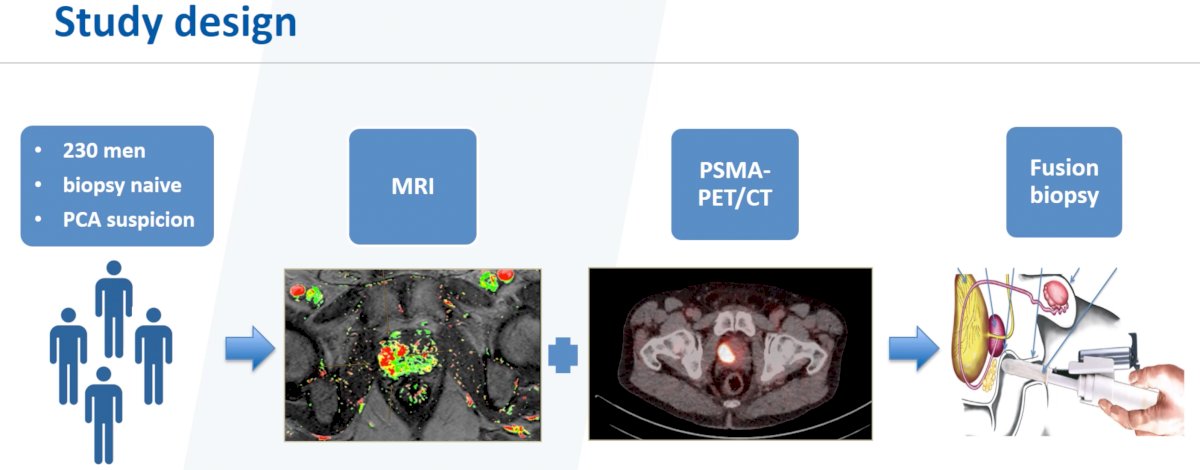
As demonstrated below, all patients will undergo both mpMRI and PSMA-PET/CT. All patients will undergo a systematic biopsy followed by a targeted biopsy of suspicious lesions (MRI and/or PSMA-PET/CT). Biopsy specimens will be evaluated for tumor grade, tumor extent, and immunohistochemistry PTEN/PSMA status. Imaging and histopathologic data will be randomly presented to four blinded investigators: one group (two investigators) will receive all information from PSMA-PET/CT and MRI-guided biopsies, the other two will receive information from the “standard of care” approach of MRI and MRI-guided biopsies (systematic + targeted). After the four investigators have evaluated the available data, comparisons will be made with regards to the treatment plan, including decision for systemic versus local therapy (including modality chosen, the extent of lymphadenectomy, nerve-sparing etc.)
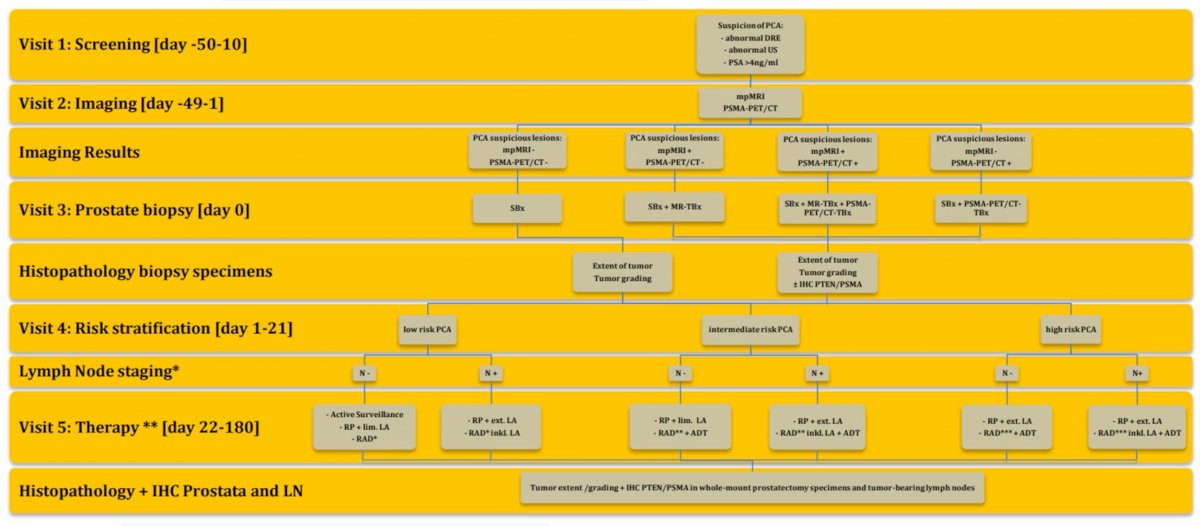
Secondary outcomes will include:
- Detection of csPCa
- Pre- and post-operative T-stage concordance
- Exploratory biomarker analyses (PSMA, PTEN)
- Test criteria
- Imaging (MRI, PSMA-PET/CT, US)
- Classifications (PIRADS v2.1, PROMISE miTNM)
- The added value of multiple biopsies
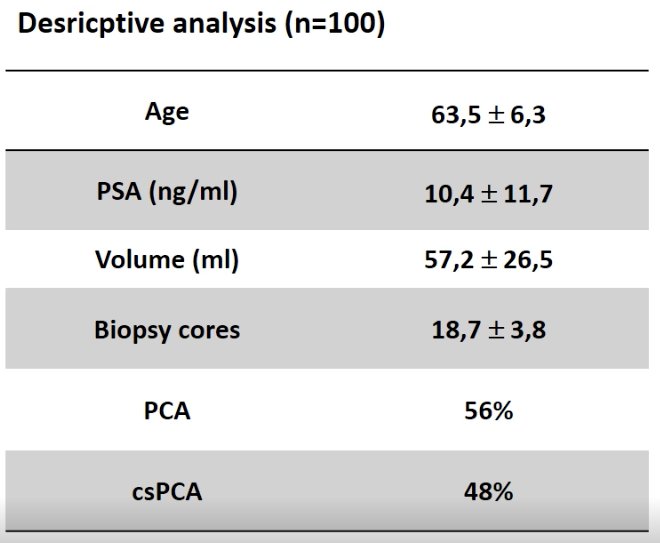
At the time of the interim analysis, data for 100 patients was available. The first patient was enrolled on March 24, 2021, and, to date, 227/230 patients have been enrolled.
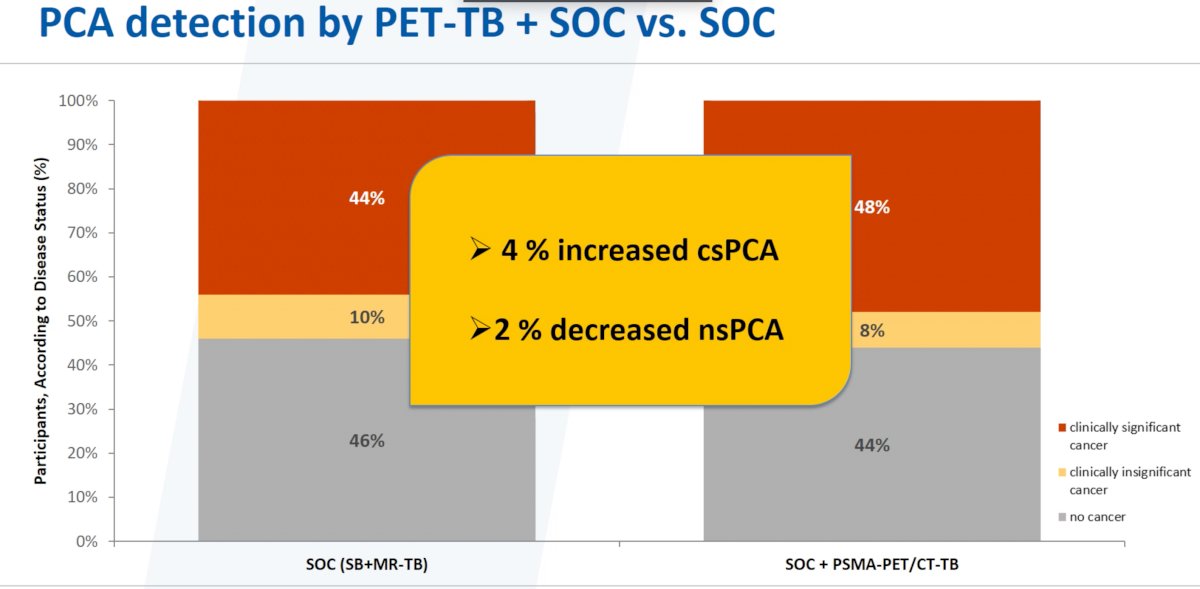
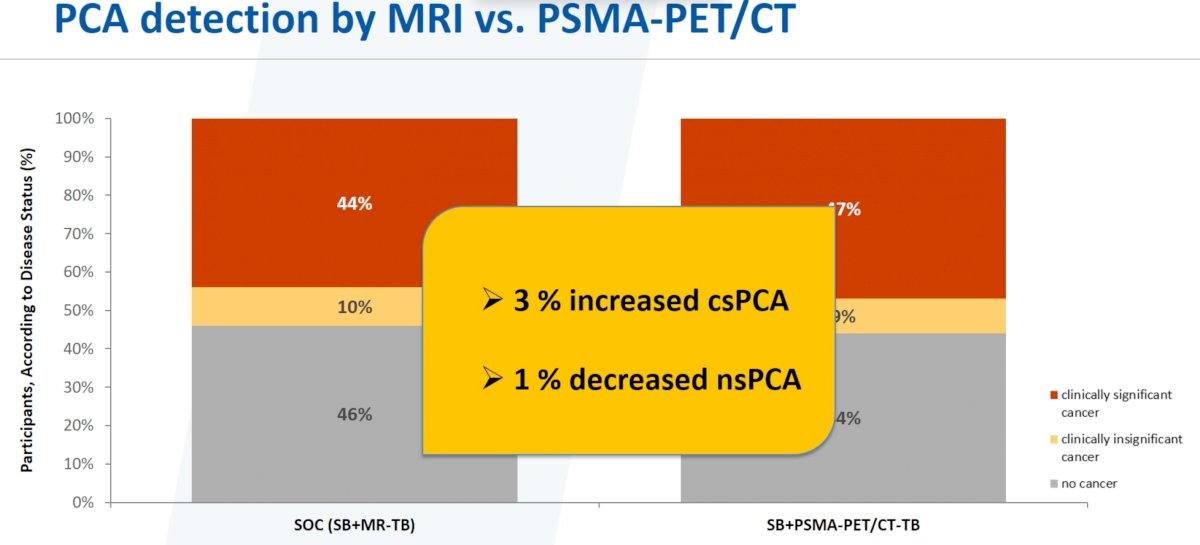
As demonstrated above, the addition of PSMA PET/CT-targeted biopsies to the standard of care practice (systematic + MR-targeted biopsies) was associated with a 4% increase in the detection of csPCa and a 2% decrease in non-significant PCa.
Conversely, had PSMA-PET/CT-targeted biopsies been performed instead of MR-targeted biopsies, this would have led to a 3% increased detection of csPCa and a 1% decrease in non-significant PCa detection.
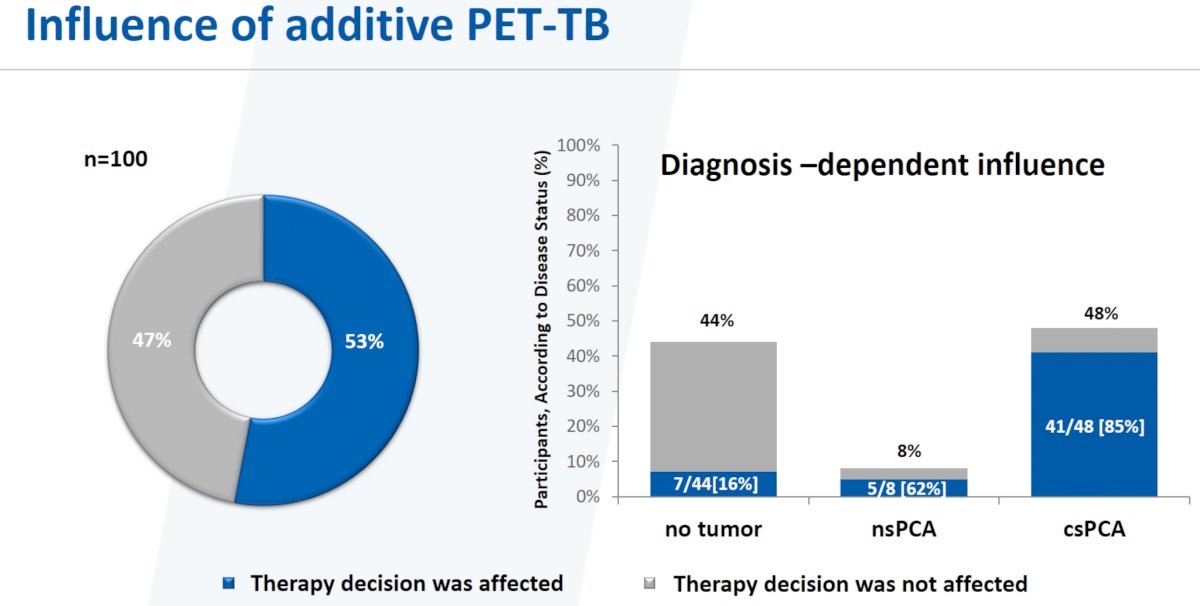
At the time of the interim analysis, the addition of a PSMA-PET/CT with targeted biopsies of positive lesions affected therapy decisions 53% of the time. This effect was most notable in the 48 patients with csPCa, with treatment decision altered in 85% of patients (41/48).
Based on these interim results, the authors concluded that the addition of a PSMA-PET/CT with targeted biopsies of positive lesions improves the detection of csPCa, is not inferior to MRI fusion biopsy, and seems to have an influence on management plans. However, these are only interim results, with final results pending. Detailed evaluations of the other endpoints are pending.
Presented by: Philipp Krausewitz, MD, University Hospital Bonn, Department of Urology and Pediatric Urology, Bonn, Germany
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Association of Urology (EAU) 38th annual congress held in Milan, Italy between March 10th and 13th, 2023


