(UroToday.com) The 2023 European Association of Urology (EAU) annual congress held in Milan, Italy between March 10th and 13th, 2023 was host to a plenary session addressing the “right management” of prostate cancer patients in the early detection and active surveillance settings. Dr. Eva Comperat discussed the minimal requirements for biopsy histopathology reporting within the context of active surveillance patient management in 2023.
The aim of the prostate biopsy is to diagnose/rule out prostate cancer. As such, uniformity of reporting using the modified Gleason Score for prostate cancer is critical. The information provided by the pathologist is critical for clinical decision-making, whereby pathology gives information needed to influence the treatment strategy (e.g. surgery, radiotherapy, active surveillance). This is only possible via communication between the clinician performing the biopsy/managing the patients and the pathologist. The clinical setting/background is critical for the pathologist’s decision-making process. For example, when a patient has received neoadjuvant hormonal therapy, this may give the tumor an appearance of a higher grade than what is truly present. As such, the pathologist needs to be aware of the relevant clinical history.
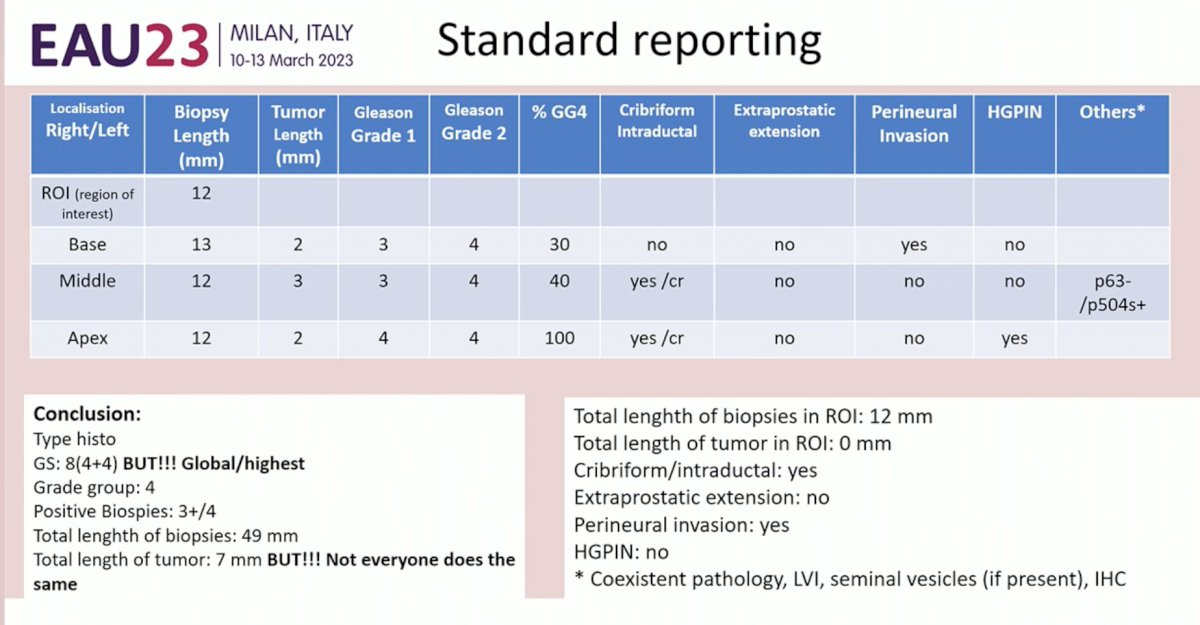
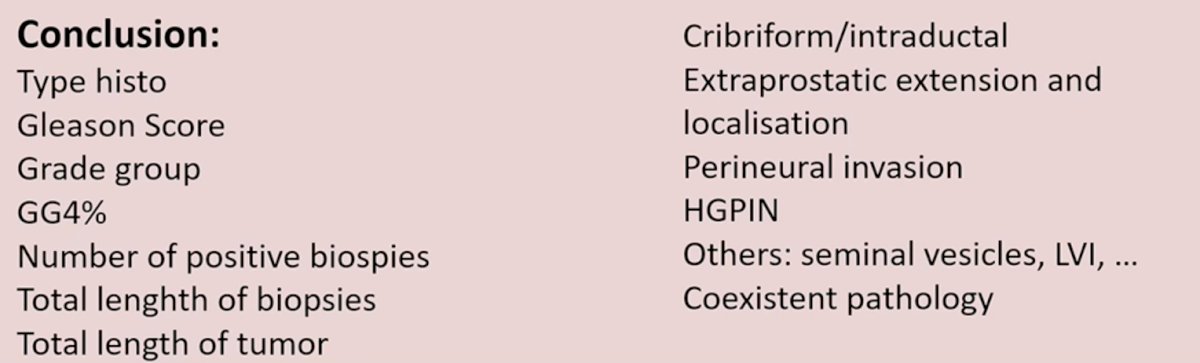
Governing bodies such as the International Collaboration on Cancer Reporting (ICCR) have set out guidelines to unify the reporting of cancer pathology. Current standard reporting of prostate biopsy pathologic specimens includes the following parameters:
- Tumor localization
- Biopsy length
- Tumor length
- Gleason Grade (1 and 2)
- % Gleason Pattern 4
- Presence/absence of cribriform/intraductal carcinoma
- Presence/absence of extraprostatic extension
- Presence/absence of perineural invasion
- Presence/absence of high-grade prostatic intraepithelial neoplasia (HGPIN)
Some pathologists report an overall Gleason Score in the summary, whereas others report the highest Gleason Score observed.
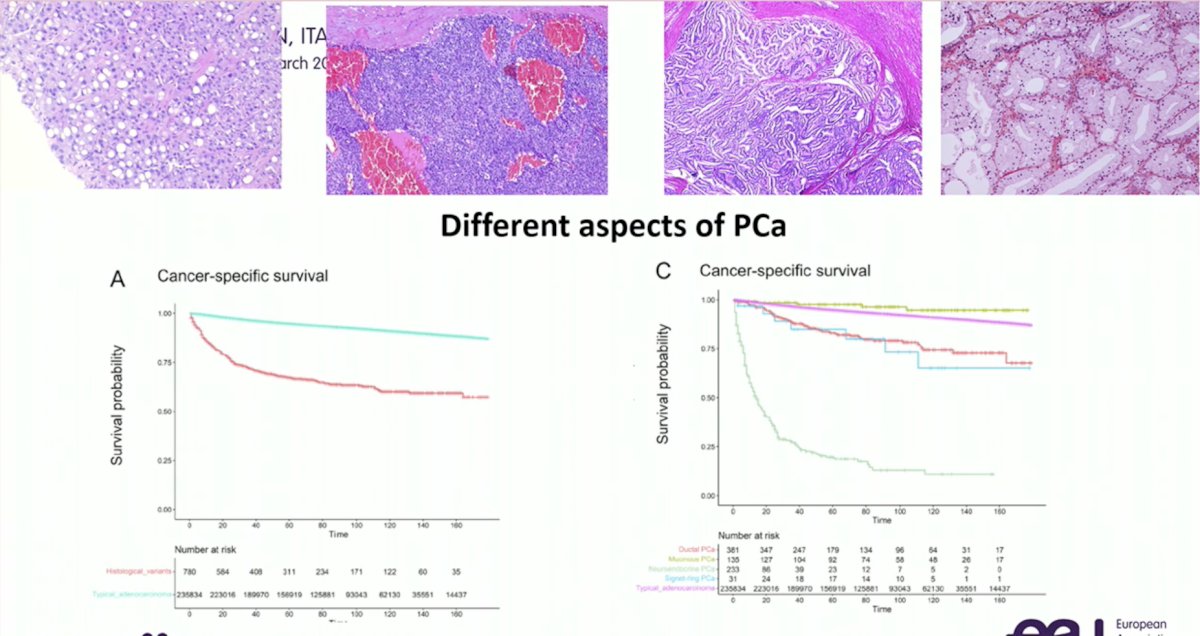
Although acinar prostatic adenocarcinoma accounts for approximately 95% of all prostate cancers, we should not assume that all such lesions are adenocarcinomas, and thus this should be explicitly reported. As seen in the Kaplan Meier curves below, cancer-specific survival is highly dependent on whether prostate cancer tumors are typical adenocarcinomas versus not:
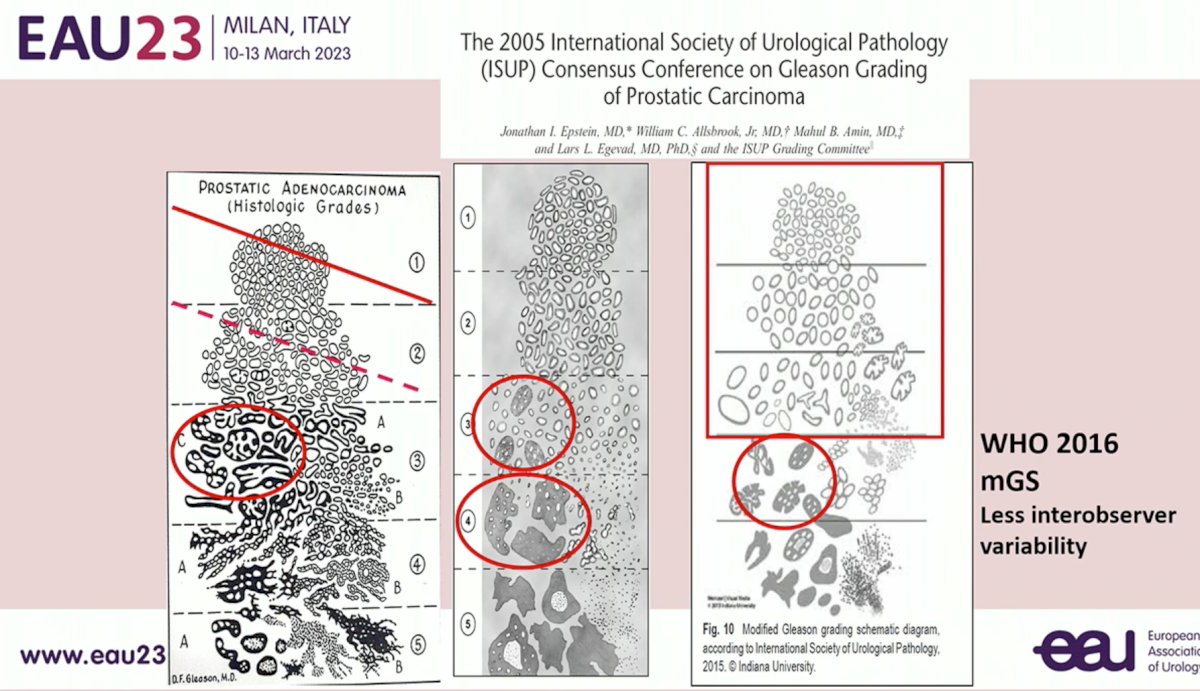
Uniformity of Gleason Score reporting across readers is critical, with the most recent modified Gleason Score reporting from the WHO 2016 update demonstrating less interobserver variability:
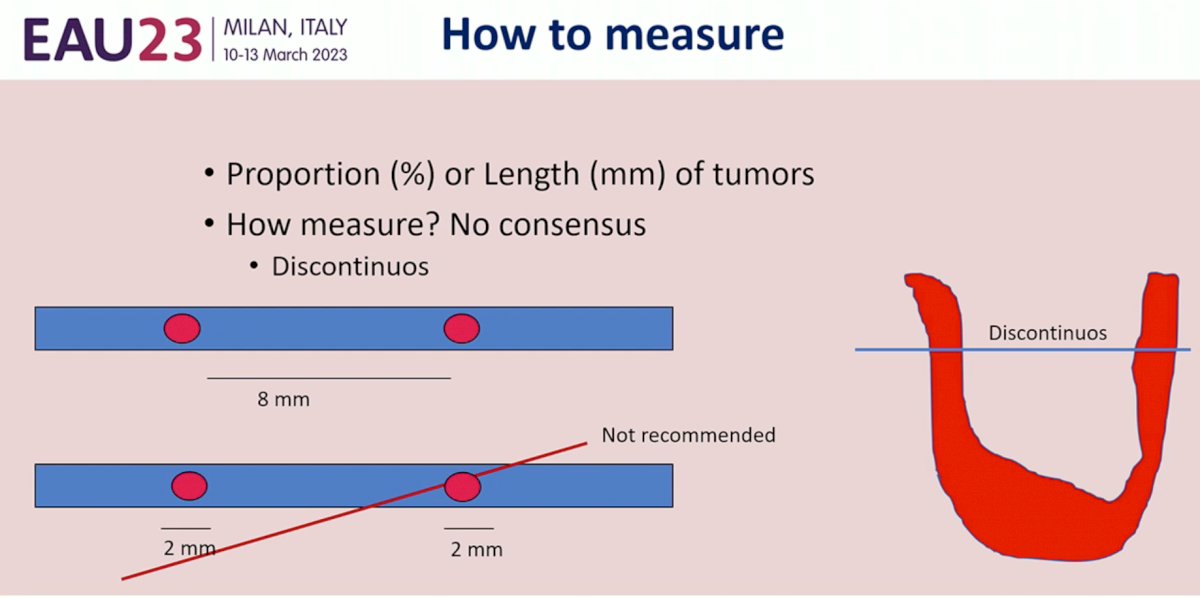
How do we measure the length of tumor? Currently, there is no consensus whether the proportion (%) or length (mm) of tumors should be reported.
As patients with cribriform pattern and intraductal prostate cancer have increased odds of biochemical relapse, extraprostatic extension, positive margins, metastases, and cancers-specific mortality hazards, awareness of whether cribriform/intraductal pattern is present is critical, and, thus, should be reported in each pathology report. However, problems relating to reproducibility and definition remain present. Furthermore, Dr. Comperat questioned whether patients with such lesions should undergo reflex genetic testing due to the increase incidence of HRR and MSI mutations in patients with such patterns.
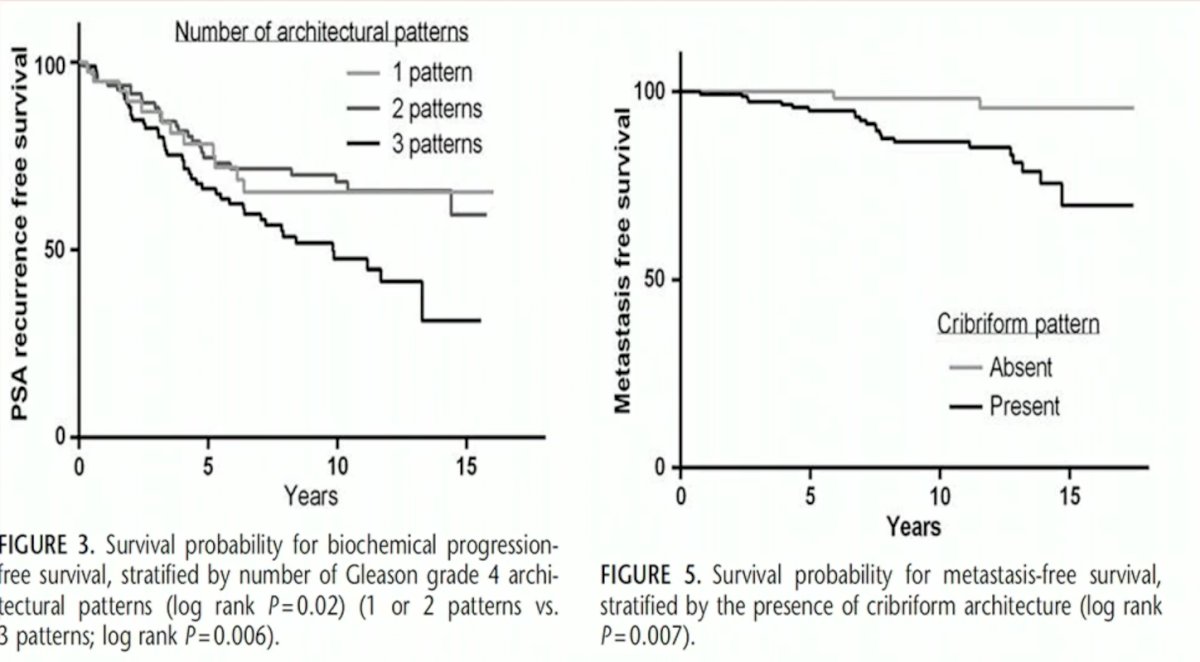
Next, Dr. Comperat addressed the issue of reporting the % of Gleason Pattern 4 disease. As numerous studies have demonstrated progressively worse outcomes as the % increases, and patients with low (i.e. <10%) % pattern 4 disease may be suitable candidates for active surveillance, the reporting of this variable is critical for clinical decision making.
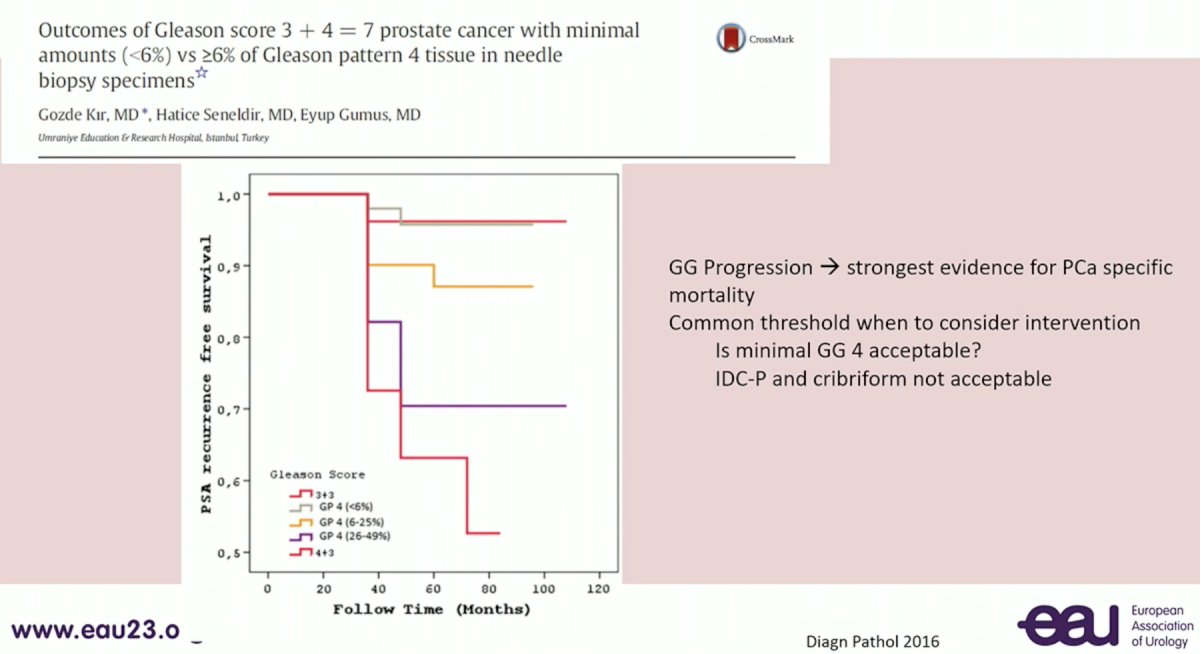
Dr. Comperat concluded her presentation with the following take home messages:
- Ideally standardized reports are the way moving forward, however consensus for which variables to report is not there yet
- Clinical information and previous treatment received are critical for helping pathologists evaluate/process/report specimens
- Localization of the biopsy is key
- Place one biopsy specimen per container
- In her opinion, the following variables should be included in each report:
Presented by: Eva Comperat, MD, PhD, Chair of Uropathology at the Medical University of Vienna and Head of the Department of Pathology at the L'Assistance Publique-Hôpitaux de Paris, Hôpital Tenon, Sorbonne University, Paris, France
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Association of Urology (EAU) Annual Meeting, Milan, IT, Fri, Mar 10 – Mon, Mar 13, 2023.
Related Content: EAU 2023: How to Manage Active Surveillance in 2023 - Do We Need a Genetic Biopsy Test to Promote as in GG2 Cancers?


