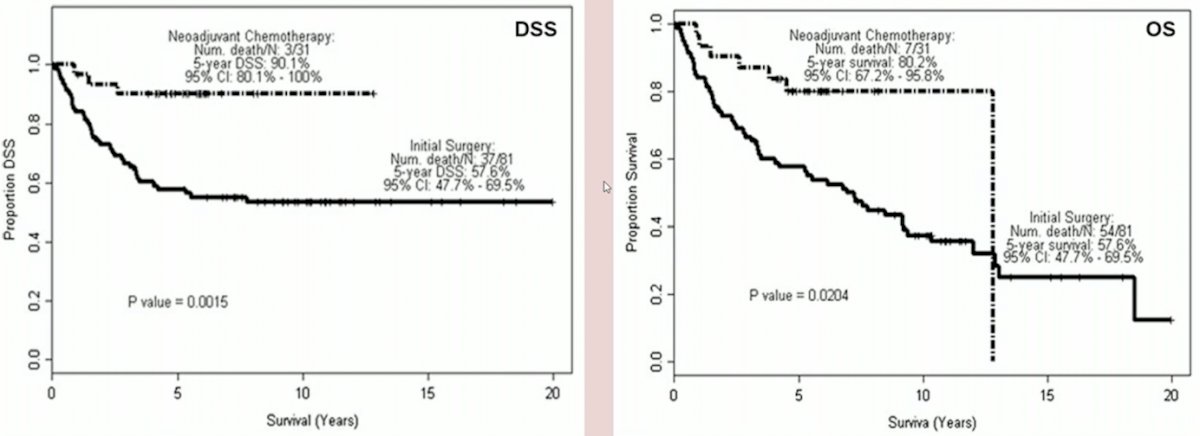
(UroToday.com) The 2023 EAU annual meeting included an EAU guideline session on effective treatment in upper tract urothelial tumors, featuring a state-of-the-art presentation by Dr. Thomas Seisen discussing the evidence for neoadjuvant treatment before radical nephroureterectomy. Dr. Seisen started with a historical perspective of neoadjuvant chemotherapy prior to radical nephroureterectomy, highlighting that the first study reported in 1995 assessed 15 patients, including a complete response in 2/15 (13%) patients. A more contemporary assessment of the MD Anderson Cancer Center experience in 20141 found that neoadjuvant chemotherapy was associated with both improved disease free survival (p = 0.0015) and overall survival (p = 0.0204):
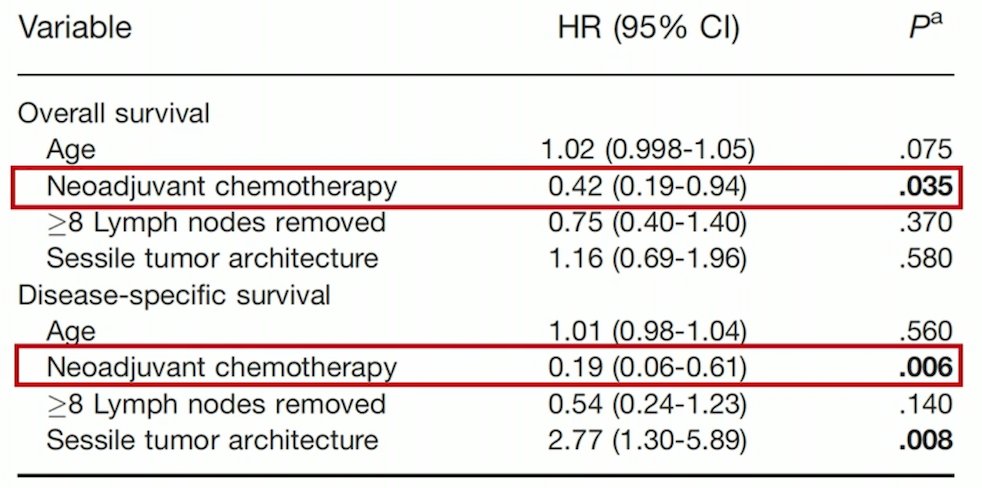
Additionally, in this study, receipt of neoadjuvant chemotherapy was predictive of overall and disease specific survival on multivariable analyses:
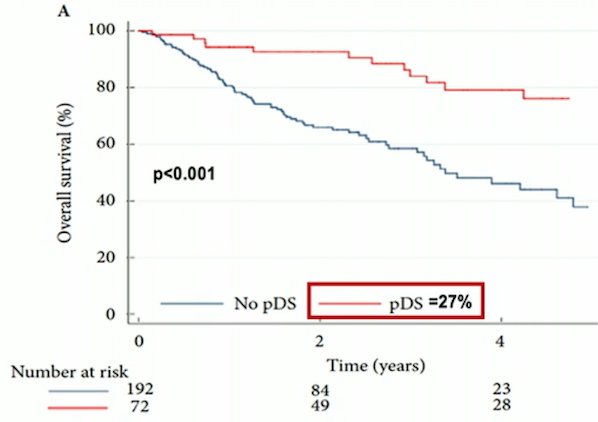
These initial encouraging results from large institutional analyses have also been corroborated at the population level. In the National Cancer Database, receipt of neoadjuvant chemotherapy was associated with pathological downstaging (OR 19.8, 95% CI 11.8-33.5), and those patients with pathological downstaging also had improved overall survival (p < 0.001):
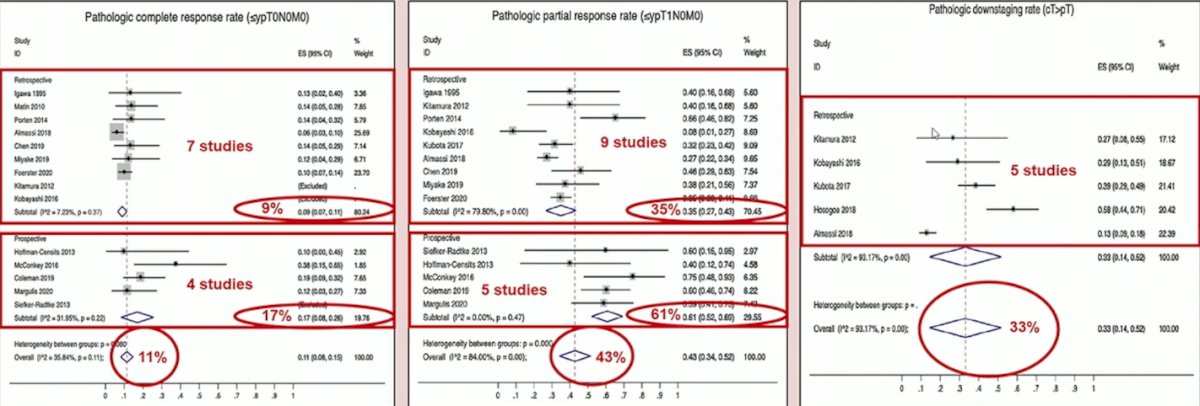
Loew and colleagues2 previously performed a systematic review and meta-analysis assessing neoadjuvant and adjuvant therapy for upper tract urothelial carcinoma. They found that for neoadjuvant chemotherapy, the pooled pathologic complete response rate (≤ypT0N0M0) was 11% (n = 811) and pathologic partial response rate (≤ypT1N0M0) was 43% (n = 869), both across 14 studies. Across six studies, the pooled HRs were 0.44 (95% CI 0.32-0.59, p < 0.001) for overall survival and 0.38 (95% CI 0.24-0.61, p < 0.001) for cancer-specific survival in favor of neoadjuvant chemotherapy. For adjuvant chemotherapy, there was a benefit in overall survival (pooled HR 0.77; 95% CI: 0.64-0.92, p = 0.004, 7,983 patients), cancer specific survival (pooled HR 0.79; 95% CI: 0.69-0.91, p = 0.001, 5659 patients), and disease-free survival (pooled HR 0.52; 95% CI: 0.38-0.70, 602 patients). A summary of these findings is as follows:

Recently, there is now phase II evidence supporting the use of neoadjuvant chemotherapy for high risk upper tract urothelial carcinoma. Margulis and colleagues3 assessed patients with high grade upper tract urothelial carcinoma in whom nephroureterectomy was planned and subsequently assigned patients to 4 neoadjuvant chemotherapy cycles of accelerated MVAC in those with baseline creatinine clearance greater than 50 ml/minute or gemcitabine and carboplatin in those with creatinine clearance 30-50 ml/minute or less. Of the 29 patients eligible for accelerated MVAC, 80% completed all planned treatments, 3 (10.3%) achieved ypT0N0 and 1 achieved ypT0Nx for a pathological complete response in 13.8% (90% CI 4.9-28.8). The grade 3-4 toxicity rate was 23% in the accelerated MVAC arm with no grade 5 events.
More recently, in early 2023, was the publication of a multicenter phase II clinical trial of gemcitabine and cisplatin as neoadjuvant chemotherapy for patients with high-grade upper tract urothelial carcinoma.4 There were 57 eligible patients with defined criteria for high-risk localized upper tract urothelial carcinoma that received four cycles of split-dose gemcitabine + cisplatin before surgical resection and lymph node dissection. Overall, 36 (63%) patients demonstrated pathologic response (95% CI, 49 to 76), and a complete pathologic response (ypT0N0) was noted in 11 patients (19%):
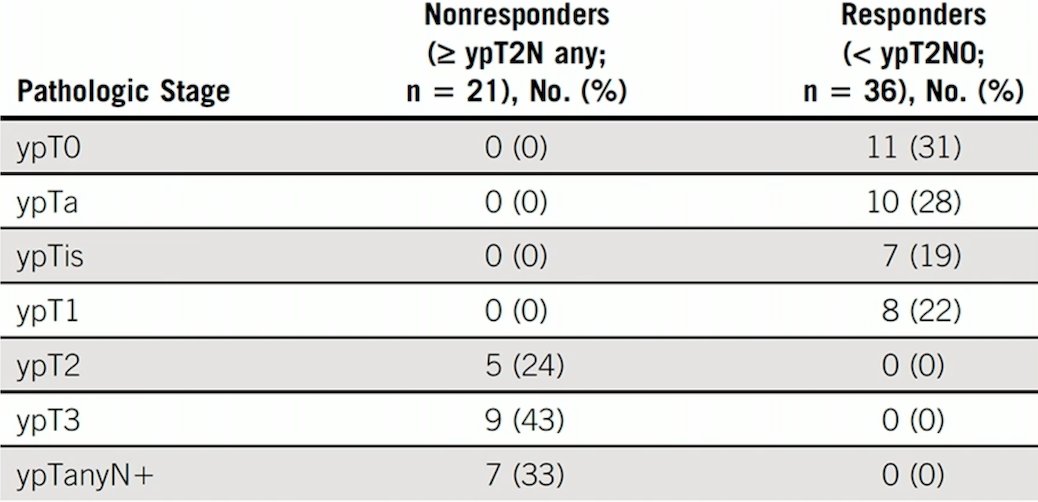
Additionally, with a median follow up of 3.1 years, 2- and 5-year PFS rates were 89% (95% CI, 81 to 98) and 72% (95% CI, 59 to 87), while 2- and 5-year OS rates were 93% (95% CI, 86 to 100) and 79% (95% CI, 67 to 94), respectively:
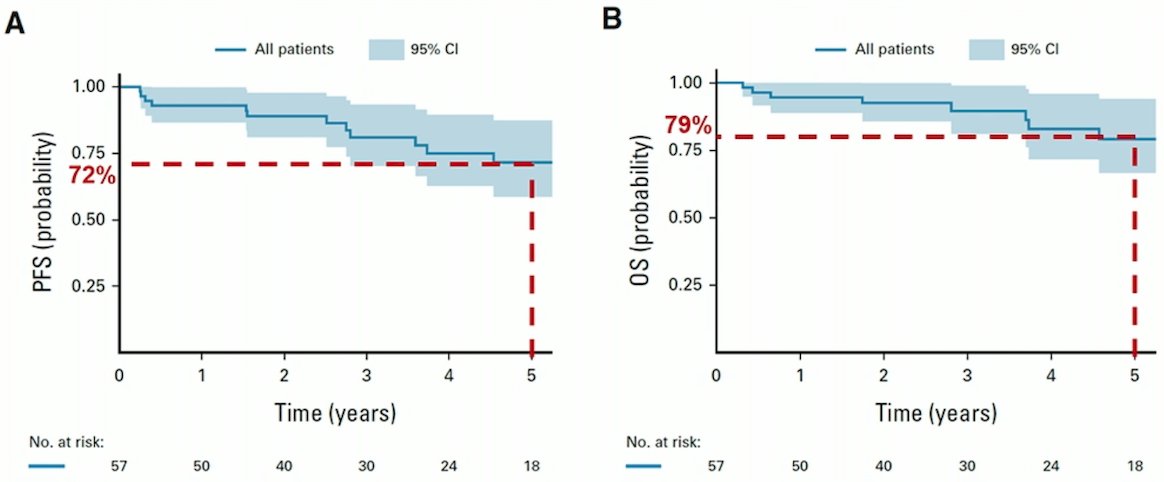
Dr. Seisen also highlighted the iNDUCT trial, a phase 2 study of neoadjuvant immunochemotherapy using durvalumab, with the following trial design:
Dr. Seisen concluded his presentation discussing the evidence for neoadjuvant treatment before radical nephroureterectomy with the following take-home messages:
- Comparative effectiveness research favors the use of neoadjuvant chemotherapy for high-risk upper tract urothelial carcinoma with all retrospective studies available in the literature showing better pathological outcomes and prolonged survival
- Prospective studies also support the use of neoadjuvant chemotherapy, although no comparative study is currently available
- Patient selection is key and remains the biggest challenge
- Ongoing studies are assessing the role of neoadjuvant immunochemotherapy
Presented by: Thomas Seisen, MD, Academic Hospital Pitie-Salpetriere, Sorbonne Universite, Paris, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 European Association of Urology (EAU) 38th annual congress held in Milan, Italy between March 10-13, 2023
References:
- Porten S, Siefker-Radtke AO, Xiao L, et al. Neoadjuvant chemotherapy improves survival of patients with upper tract urothelial carcinoma. Cancer 2014 Jun 15;120(12):1794-1799.
- Leow JJ, Chong YL, Chang SL, et al. Neoadjuvant and Adjuvant Chemotherapy for Upper Tract Urothelial Carcinoma: A 2020 Systematic Review and Meta-Analysis, an Future Perspectives on Systemic Therapy. Eur Urol. 2021 May;79(5):635-654.
- Margulis V, Puligandla M, Trabulsi EJ, et al. Phase II trial of neoadjuvant systemic chemotherapy followed by extirpative surgery in patients with high grade upper tract urothelial carcinoma. J Urol. 2020 Apr;203(4):690-698.
- Coleman JA, Yip W, Wong NC, et al. Multicenter phase II clinical trial of gemcitabine and cisplatin as neoadjuvant chemotherapy for patients with high-grade upper tract urothelial carcinoma. J Clin Oncol. 2023 Mar 10;41(8):1618-1625.


