(UroToday.com) The 2023 EAU annual meeting included an EAU guideline session on effective treatment in upper tract urothelial tumors, featuring a state-of-the-art presentation by Dr. Maria De Santis discussing immunotherapy in locally advanced and metastatic upper tract urothelial carcinoma. Dr. De Santis notes that there are specific challenges for the treatment of upper tract urothelial carcinoma, namely that when considering perioperative chemotherapy, cisplatin eligibility declines following nephroureterectomy, thus there is a need for cisplatin-free options: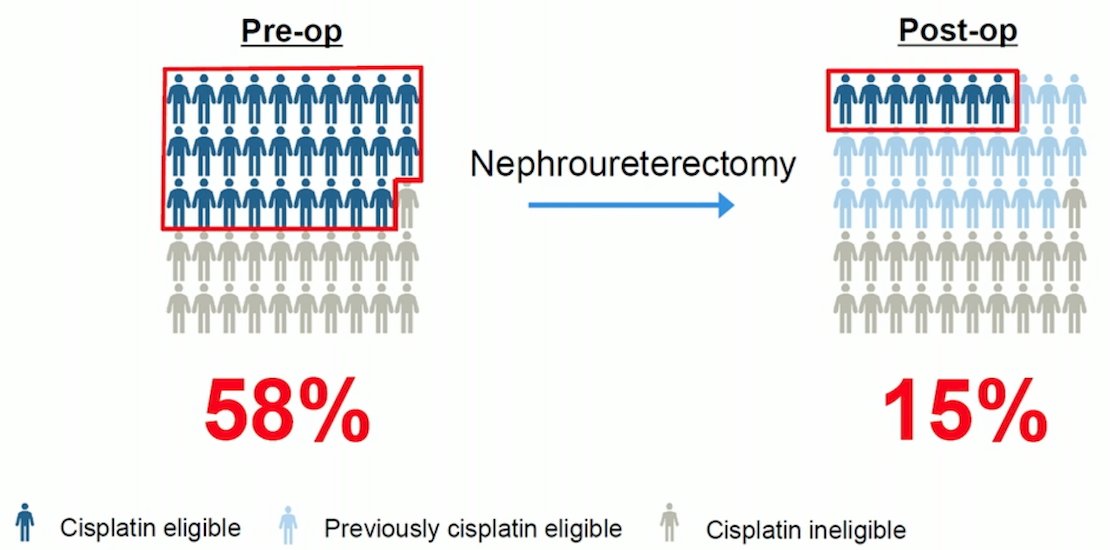
Furthermore, is important to consider if upper tract urothelial carcinoma is different from bladder urothelial carcinoma. In a pooled analysis of the EORTC 30924, 30986, and 30987 trials (pre-immunotherapy era), the location of the primary tumor in urothelial carcinoma patients did not seem to markedly impact PFS or OS, however the findings may vary according to treatment. Upper tract urothelial carcinoma mutational signature profiles are important, centering on whether tumors are EpiC-low cluster or EpiC-high cluster: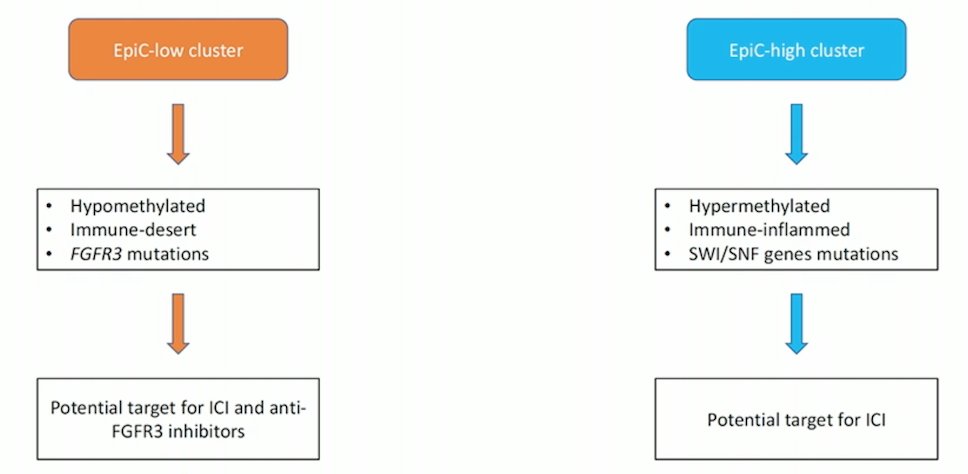
Dr. De Santis then discussed the current immunotherapy data in upper tract urothelial carcinoma, all of which is derived from studies assessing immunotherapy in locally advanced or metastatic urothelial carcinoma. As follows is a summary of the trials reporting data of upper tract urothelial carcinoma patients in the first-line setting: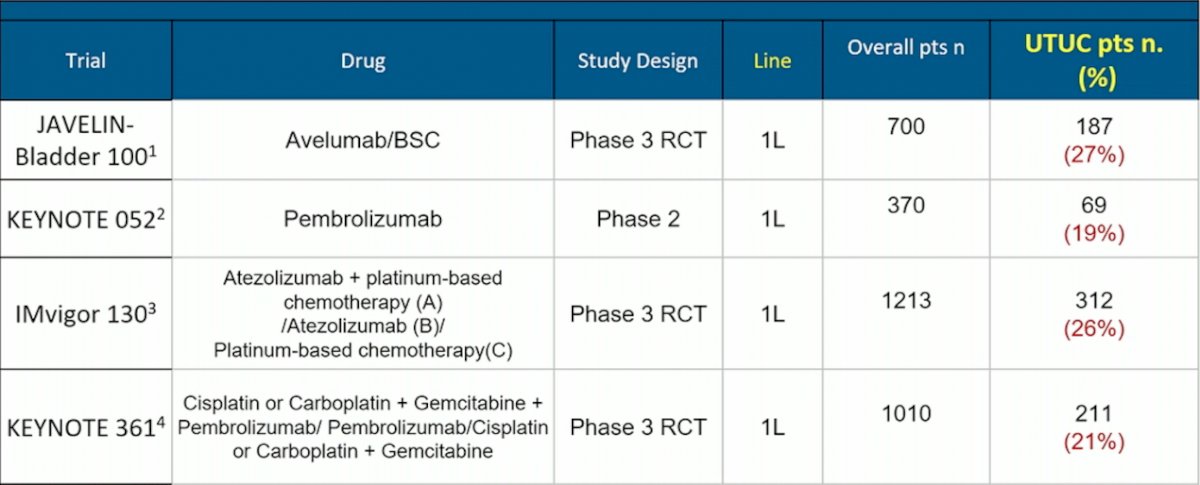
Specifically, the JAVELIN Bladder 100 trial [1] enrolled 700 patients of advanced urothelial carcinoma after progression on platinum-based chemotherapy, of which 187 (26.7%) had upper tract urothelial carcinoma. The subgroup analysis showed that the HR for OS in this trial was 0.62 (95% CI 0.48-0.80) for bladder urothelial carcinoma and HR 0.89 (95% CI 0.58-1.37) for upper tract urothelial carcinoma. In the KEYNOTE-052 trial assessing first-line pembrolizumab, 19% of patients were upper tract urothelial carcinoma, with a 22% objective response rate.
As follows is a summary of the trials reporting data of upper tract urothelial carcinoma patients in the second-line setting:

Importantly, the KEYNOTE-045 trial2 assessing pembrolizumab in the second-line setting, did include a subgroup analysis for patients with upper tract urothelial carcinoma. Patients with tumors in the lower urinary tract demonstrated a survival benefit (HR 0.77, 95% CI 0.60 to 0.97), and although the numbers were small (10% of the overall trial population), patients with tumors in the upper tract also did quite well with second-line pembrolizumab (HR 0.53, 95% CI 0.28 to 1.01).
The final portion of Dr. De Santis’ talk focused on special patient subgroups of interest. She notes that mismatch repair (MMR) deficiency, due to pathogenic variants in MLH1, MSH2, MSH6, and PMS2, as well as microsatellite instability, are known for the development of Lynch Syndrome associated carcinogenesis. Lynch-associated urothelial cancer may be more common than previously known or understood, given that point of care testing strategies identifies upper tract urothelial carcinoma in 1.5-2% of new cases of Lynch Syndrome. Additionally, there are promising response rates to immune checkpoint inhibitors, which are similar to conventional urothelial carcinoma.
Dr. De Santis concluded her presentation discussing immunotherapy in locally advanced and metastatic upper tract urothelial carcinoma with the following take-home messages:
- Challenges for the treatment of upper tract urothelial carcinoma include renal function decline after surgery
- Immunotherapy can be given despite a low GFR
- Differences in biology and distinct molecular subgroups may account for difference in response to immunotherapy as compared to bladder urothelial carcinoma
- Data on immunotherapy for upper tract urothelial carcinoma are scarce: upper tract urothelial carcinoma makes up 20-30% of subgroups in urothelial carcinoma trials, and benefit varies by trials and uncertainty remains due to small numbers of upper tract urothelial carcinoma subgroups
- Selected upper tract urothelial carcinoma patients may derive major benefit from immunotherapy, such as patients with Lynch syndrome, MSI-H/MMRd, and EpiC-high clusters
Presented by: Maria De Santis, MD, PhD, Charité University Hospital, Berlin, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 European Association of Urology (EAU) Annual Meeting, Milan, IT, Fri, Mar 10 – Mon, Mar 13, 2023.
References:
- Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2020 Sept 24;383(13):1218-1230.
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017;376(11):1015-1026.


