(UroToday.com) The treatment landscape in the first-line therapy of metastatic renal cell carcinoma (mRCC) has rapidly evolved over the past 3 years. While the proliferation of treatment options has provided ample treatment choice, deciding among these can be difficult. In a presentation at the European International Kidney Cancer 2021 Virtual Annual Meeting, Dr. Brian Rini presented on how to choose between VEGF + immune checkpoint inhibition or combined immune checkpoint inhibition with ipilimumab and nivolumab.
Dr. Rini began by highlighting data from an analysis of the IMmotion151 cohort which examined clusters of disease characteristics, highlighting that most patients have tumors driven by angiogenic (cluster 1 and 2) and inflammatory (cluster 4 and 5, and potentially 7) pathways.
These mechanisms of action suggest both the rationale for current approaches with small molecule tyrosine kinase inhibitors (targeting angiogenic pathways) and with immune checkpoint inhibitors (targeting inflammatory pathways) but also highlights that a subset of tumors are not necessarily appropriately targeted by current therapies.
Dr. Rini then moved to summarize what he referred to as “major trials” which have shown a survival advantage in advanced kidney cancer, including CheckMate214, KEYNOTE-426, CheckMate9ER, and CLEAR.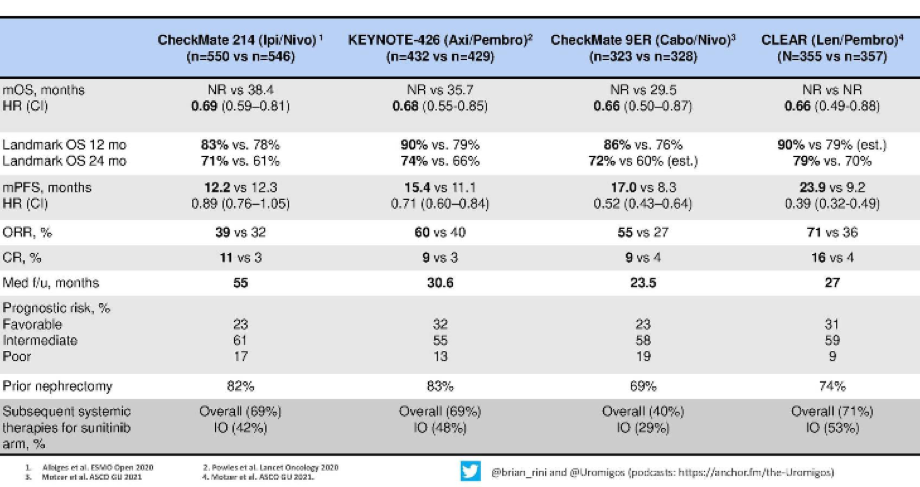
He emphasized that each of these trials has shown a clear benefit for combination approaches with an immunotherapy backbone, compared to sunitinib with a relative benefit of 30-35%. Further, examining landmark survival at both 12 months and 24 months, Dr. Rini suggested that these results were again fairly comparable, though potentially with better the combination of lenvatinib and pembrolizumab.
Moving to more tumor-response based assessments, Dr. Rini highlighted that regimes containing a tyrosine kinase inhibitor (all but nivolumab/ipilimumab) have better objective response rate and progression-free survival, compared to the combination of nivolumab/ipilimumab.
Complete response rates, potentially the holy grail in systemic therapy in which we can actually consider cure, are approximately 10% with each treatment approach. Dr. Rini highlighted that the rate is somewhat higher in the CLEAR trial with lenvatinib and pembrolizumab though it is unclear whether this represents a truly higher rate or just the high end of a confidence interval around the same overall effect.
Dr. Rini then presented updated data on progression-free survival and duration of response from the CheckMate214 trial, with follow-up to 42 months. He emphasized that, among patients receiving nivolumab/ipilimumab, approximately one-third have long-term progression-free survival at 3 and a half years. As a result, there is clearly a subset of patients susceptible to immunotherapy. Further, this highlights one of the characteristics of immune checkpoint inhibitors, namely their durability. Notably, these effects were observed across risk groups.
Dr. Rini then highlighted a variety of pros and cons with respect to the use of IO and TKI combinations as compared to combination PD-1/CTLA-4 approaches. Notably, the combination of IO and TKI has greater tumor shrinkage though the durability of responses has yet to be demonstrated. Further, these combinations have the potential for acute and chronic TKI toxicity. Additionally, the combination of PD-1/CTLA-4 targeting has the potential to have a treatment-free interval. Ongoing analyses of the KEYNOTE-426 data will help with learning about the ability to have off-therapy periods for patients treated with IO/TKI combinations.
Current SITC guidelines recommend the use of immunotherapy-based regimes across all risk groups, and in patients with sarcomatoid histology.
Circling back to the initial data he presented from the IMmotion151 trial, Dr. Rini highlighted that when tumor clusters are assessed across risk groups (whether by MSKCC or IMDC risk group), nearly all clusters are represented within each risk group. Notably, a greater proportion of patients with favourable disease fall into the angiogenic cluster. However, these data highlight the heterogeneity within risk groups and highlight the importance of ongoing work to provide truly personalized therapy.
To do so, Dr. Rini described a planned trial in which patients will be assigned therapy on the basis of their tumor cluster: those with an angiogenic signature (clusters 1 and 2) will receive the combination of IO/TKI, those with an inflammatory signature (clusters 4, 5, and 7) will receive an IO/IO combination, while those in clusters 3 and 6 will receive an investigators choice of regime. This biomarker driven approach to treatment will be compared to current treatment selection approaches based on physician preference and risk group classification.
Dr. Rini concluded that renal cell carcinoma, based on its angiogenic and inflammatory characteristics, is a disease responsive to both anti-VEGF and IO therapies. In the past few years, immunotherapy based doublet approaches have transformed the landscape of first-line mRCC treatment and all patients should be offered one of these approaches as sunitinib is no longer the standard of care.
Presented by: Brian Rini, MD, Professor of Medicine, Chief of Clinical Trials, Vanderbilt University Medical Center
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center Contact: @WallisCJD on Twitter during the 2021 European International Kidney Cancer Symposium (EIKCS), April 23-24, 2021


