(UroToday.com) In the tenth session of the 2022 International Kidney Cancer Symposium (IKCS): Europe meeting focusing on biomarker discovery in renal cell carcinoma (RCC), Dr. Elfriede Nößner presented on the role and potential of vaccines and cellular therapies.
She began by highlighting the differences between these two treatment approaches. Beginning first with vaccines, she highlighted that the goal of oncologic vaccination is to induce, restore, or reinforce T cell responses in patients with insufficient anti-tumor immunity. To do so, the process of vaccine involves an ex vivo identification and selection of antigens, which may be peptides or messenger RNA molecules. These antigens are then injected into the patients and, in situ, the patients must have the ability to raise an immune response with the T cells primed by the vaccine needing to migrate to the tumor and then kill antigen presenting tumor cells. In contrast, adoptive cell therapies do not require this patient level immune response and can thus work in an immunosuppressed patients. Adoptive cell therapies function by transferring T cell or natural killer (NK) cells directly into a patient for a “ready-made” anti-tumor response. These therapies are prepared ex vivo by engineering these cells with T cell receptor or chimeric antigen receptors targeted to the identified tumor antigen.
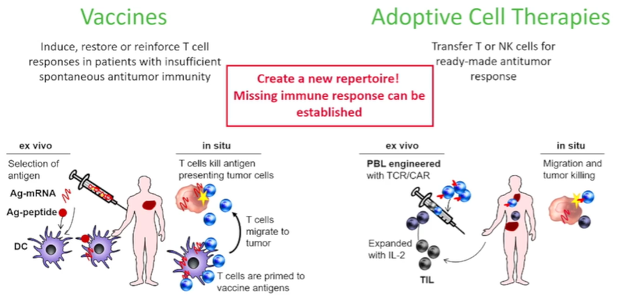
However, in renal cell carcinoma (as with many other solid tumor malignancies), we are limited by our ability to identify the targets and antigens for each of these approaches. In the context of vaccines, we may consider peptides, proteins, mRNA-antigens, recombinant vector-antigen, antigen-loaded dendritic cells, whole-cell tumor, or dendritic cell and tumor cell fusions. For adoptive cell therapies, options, broadly speaking, include native antitumor reactivity (such as tumor-infiltrating lymphocytes (TILs), lymphokine-activated killer cells (LAKs), cytokine-induced killer cells (CIKs), or natural killer cells) and engineered single antigen specific treatments including CAR-T cells and natural killer cells or TCR-T cells. Each of these have specific characteristics. CAR-T and NK based treatments are not MHC-restricted but are limited to surface proteins. In contrast, TCR-T is MHC-restricted but are not limited to surface proteins. There is a further question of which T cell subset should be used.
Dr. Nobner then considered the question of which specific antigens should be targeted. To date, there have been many antigens identified, all of which have been used in vaccination trials in different formats and approaches. Importantly, these have been used in a variety of different therapeutic formats. While these specific antigens have garnered much work, she emphasized that utilizing tumor cells (whether as whole cells, with mRNA, gp96-peptide complexes, lysates, or as a dendritic cell fusion) doesn’t necessarily require knowing the specific antigen.
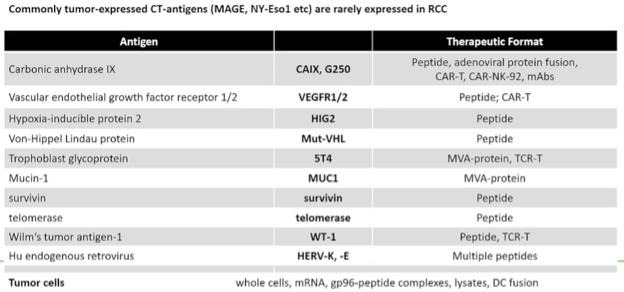
While many of these have been tested in trials, there have been four phase II trials performed examining tumor-derived gp96, peptides, recombinant MVAA-ST4, and autologous dendritic cells transfected with CD40L and tumor mRNA. Unfortunately, all of these completed vaccination trials have been unsuccessful. Interestingly, some of these trials have shown a vaccination effect with the development of anti-vector immunity, but without clinical benefit. In three of these trials, the vaccine was combined with sunitinib which, she suggested, may be detrimental to the efficacy of the vaccine. However, moving forward, she offered two potential future considerations: identification and use of better, stronger antigens and the use of better combination therapy partners (including potential immune checkpoint inhibitors or tumor microenvironment modulators).
Beyond these four phase III trials, there have been a host of other phase I, I/II, and II trials. Taken together, she suggested that these approaches are unlikely to be successful as stand alone therapy given the hurdles posted by the cancer immunity cycle. This process, as highlighted in the figure below, involves a process of antigen release and presentation followed by T cell activation, proliferation, trafficking, infiltration, and recognition prior to cancer cell elimination.

Given the complexity of this process, it should not be surprising that immune escape itself is complex and may involve many aspects of this cycle. Suppression of the elimination process prevents antigen release which may impair the entire process. While vaccination may overcome this by providing antigens for presentation and adoptive cellular therapies may obviate the need for T cell activation and proliferation, the processes of trafficking, infiltration, recognition, and elimination cannot be addressed by vaccination alone. Thus, she emphasized the importance of combining these treatment approaches with others. She highlighted a number of “new trials” of RCC vaccination, each of which employs combination immune checkpoint inhibition with either anti-CTLA4 or anti-PD-1/L1 therapy.
Moving from vaccination to adoptive cellular therapies, she noted differences between treatments which rely on a broad native anti-tumor reactivity (TIL, LAK, CIK, NK) and those with specific single antigen engineered specificity (TCR-T and CAR-T/NK). In the first of these cases using the native immune response, there have been a number of trials performed assessing a variety of different cell types from a variety of sources, in combination with a variety of systemic therapies including interleukin, immunotherapy, chemotherapy, and others.
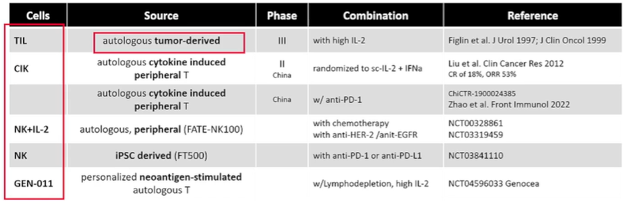
In the context of TILs, the trial from Figlin et al. demonstrated a relatively high response rate (35%) though it is somewhat unclear to what extent this was due to the co-administered IL-2. While this approach allows targeting of multiple antigens, the production process is problematic though this is improving. A number of ongoing trials are assessing the role of TILs in combination with immune checkpoint inhibitors across a number of tumor sites. A relatively newer approach is the use of neoantigen stimulated T cells: GEN-011 is an investigational, personalized treatment approach that uses ATLAS (Antigen Lead Acquisition System) to identify true immunogenic neoantigens from each patient’s tumor which may be used to stimulate and select T cells to produce the cell product ex vivo.
In contrast to these approaches, engineered T cells are an alternative.
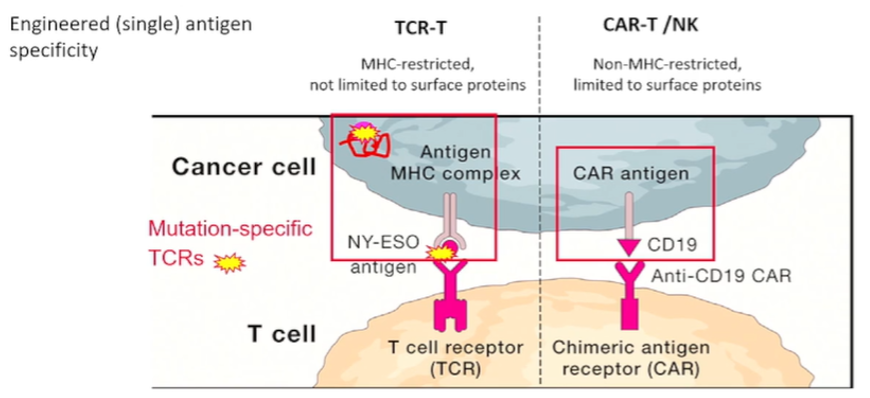
There have been a number of these tested in RCC including against CAIX, VEGFR2, CD70, c-MET, HERV-E, 4TS, and erbB2/HER2. While these have promise, Dr. Nobner suggested that the hurdles for cellular therapy will prevent it from succeeding as monotherapy for solid tumor malignancies such as RCC.
In concluding, Dr. Nobner emphasized that vaccination and adoptive cell therapy can broaden the antitumor immune response and provide some initial antitumor reactivity. However, trafficking, immunosuppressive and exhaustion will curb this activity. Thus, work to combat the immune suppression is needed. To this end, combination therapy approaches are needed and more work is required to understand the best combination partner. Additionally, as antigen choice is crucial to maximize on-target and minimize off-target effects, further work is needed in this regard as well.
Presented by: Elfriede Nößner, PhD, Helmholtz Zentrum München, Marchioninistr, München Germany

