(UroToday.com) In the tenth session of the 2022 International Kidney Cancer Symposium (IKCS): Europe meeting focusing on biomarker discovery in renal cell carcinoma (RCC), Dr. Haanen presented on approaches to understanding and overcoming both primary and secondary resistance to immune checkpoint inhibition in renal cell carcinoma (RCC).
To begin, he emphasized that primary resistance is due to resistance mechanisms that predate the initiation of therapy. Thus, these patients have primary progressive disease. In contrast, secondary resistance may be somewhat pre-existing but emerges under the pressure of treatment such that patients with initial response to therapy subsequently develop disease progression. There are both shared or overlapping as well as unique mechanisms between these two phenomena.
In the search for biomarkers to identify both primary and secondary treatment resistance, Dr. Haanen emphasized the potential of both tumor and host factors as potential sources.
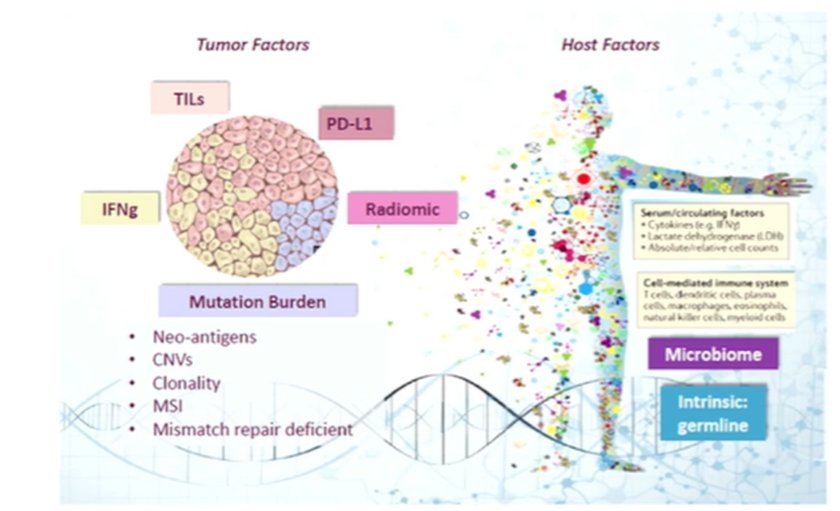
He noted that ongogenic driver mutations can be of importance in resistance to immunotherapy by affecting the infiltration of T cells into the tumor and thus mediating the tumor microenvironment.
To lay the ground work for ongoing discoveries, he highlighted the relatively limited repertoire of available markers. In kidney cancer, there is minimal clinical utility of these. In terms of PD-L1 expression, he emphasize that, despite somewhat conflicting data, overall he felt that there is no correlation with response. Further, tumor mutation burden (TMB) does not appear to predict response to immunotherapy for these patients. While for HERV that data are somewhat conflicting, he suggested that there may be an inverse correlation with response. Finally, considering TILs, he suggested that some subtypes (CD8/CD29) may have some correlation despite an overall null association.
Learning from other disease spaces, he highlighted some data from the melanoma literature in which expression of LAG-3, an immune checkpoint distinct from PD-1, is associated with poorer outcomes. As these two checkpoints are often co-expressed on tumor-infiltrating lymphocytes and may contribute to tumor-mediated T-cell exhaustion, there was a pre-clinical rationale for combined blockade with anti-LAG-3 and anti-PD-(L)1. This was assessed in the global randomized RELATIVITY-047 trial of the anti-LAG-3 relatlimab with nivolumab in melanoma. This study showed a significant improvement in progression-free survival with the combination approach and a trial of this regime is currently being explored in the neoadjuvant setting.
However, there are other antigens which may also be targeted: IL-2 has a long history in RCC and IL-2 based targeting either using different receptor targeting, or to different cell types, offers new opportunities for therapy.
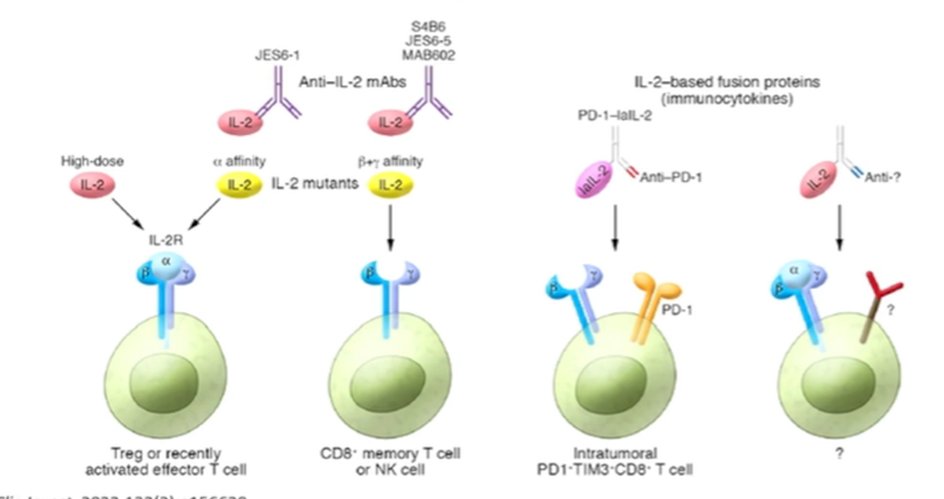
One such approach is the use of pegylated IL-2, known as bempegaldesleukin (NKTR-214). This agent gets converted to active cytokine (2-PEG-IL-2 and 1-PEG-IL-2) which binds to the beta/gamma receptor. In the phase I PIVOT-02 dose-escalation trial in combination with nivolumab, this treatment paradigm showed some promise in metastatic RCC. However, while not yet published, the phase III trial of this treatment approach in metastatic melanoma was reported to be negative according to press release and as such the program has been stopped.
There are, however, alternative ways to target the tumor microenvironment including metabolomic pathways. One such approach is the adenosine pathway in which immunosuppressive effects are seen when adenosine binds to the A2 receptor. Thus, agents such as CPI-444, PBF-509, MK-3814, and AZD4635 have been developed to block the receptor. Other agents (POM1, IPH52, MED9447, and BMS-986179) have been explored even earlier in the adenosine pathway. Dr. Haanen further noted that CD73 expression was seen in more aggressive disease (sarcomatoid and high grade histology) and had prognostic significance.
He then moved to discuss the potential role of T cells, and T cell based therapies. Building on the approach of leukapheresis with T cell expression modification and autologous transfusion, he noted that there is ongoing work on fully synthetic receptors using chimeric antigen receptor design. The antigen binding domains in these may be optimized with a single chain variable fragment that allows bypassing of MHC antigen presentation with direct activation of the T cell with cancer cell antigens. The activation domains enhance proliferation, and activation of CAR-T cells and CAR T-cell mediated tumor cell killing.
One target for such approaches is CD70 which is expressed in both primary and metastatic RCC. Its expression is driven by the loss of VHL function and correlates strongly with survival. VHL loss leads to increase in CD70 which leads to increased CD27 in serum. This may be measurable and actionable as a biomarker. He further noted recent work in allogenic CAR-T cell therapy using donor T cells with MHC class I knock out to allow for CD70 targeting using a single step multiplex editing process.
Finally, Dr. Haanen noted that the gut microbiome may play an important role in resistance to immune checkpoint inhibitors. As highlighted in a recent publication by Dr. Pal’s group in Nature Medicine, changes in the gut microbiome through the use of live bacterial supplementation may have meaningful effects on the efficacy of nivolumab and ipilimumab as first line therapy.
In summary, he concluded that better understanding the drivers of immune escape is key to developing biologically rationale combination therapy. In the context of RCC, these drivers remain poorly understood. However, there are numerous opportunities to improve outcomes including targeting of more than one immune checkpoint, combination therapy with cytokines or immunocytokines, targeting of the adenosine pathway, cellular therapies, and modulation of the gut microbiome.


