(UroToday.com) The 2022 International Kidney Cancer Symposium (IKCS) European Annual meeting included a session on surgical approaches to kidney cancer and a presentation by Dr. Axel Bex discussing how to select RCC patients for adjuvant therapy. Dr. Bex started his presentation by noting that the adjuvant therapy for RCC has been a contentious disease space over the last several years, noting that several negative trials, such as ASSURE,1 dampened optimism for adjuvant therapy. However, amongst the negative trials, the S-TRAC trial2 demonstrated a 3-year DFS rate of 64.9% for those receiving sunitinib versus 59.5% for those receiving placebo, as well as a 5-year DFS rate benefit (sunitinib 59.3% versus placebo 51.3%). Given the potential toxicity for adjuvant therapy, there is also concern for risk of overtreatment, considering that:
- 40% of patients were disease free at 84 months in ASSURE
- 40% of patients were disease free at 7-8 years in S-TRAC
- 60% of patients were disease free at 4 years in PROTECT3
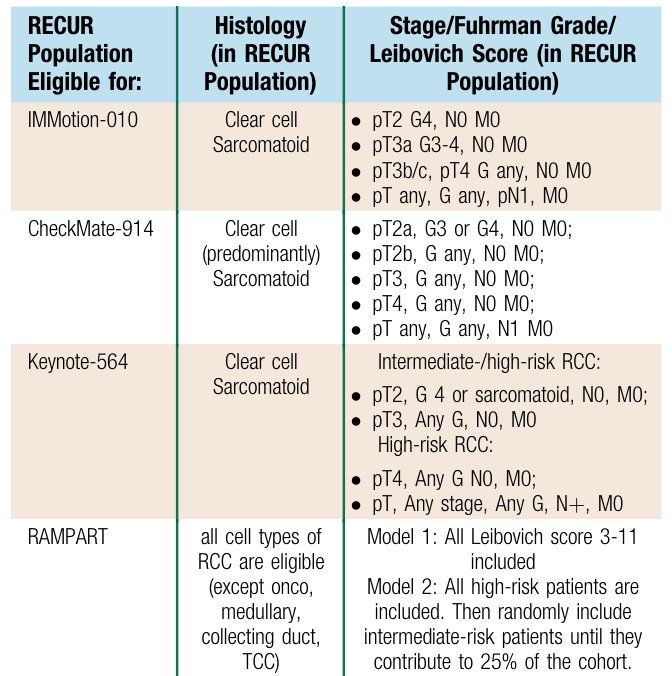
On the contrary, Dr. Bex notes that there is a risk of being too selective. In the IMmotion 010 trial 15.9% of patients were eligible, 25.5% in CheckMate 914, 21.7% in KEYNOTE-564, and 45.7% in RAMPART.4 Ultimately, TNM classification is the risk-assessment of choice for delineating inclusion criteria:
Dr. Bex then discussed the pivotal KEYNOTE-564 adjuvant pembrolizumab trial, first presented by Dr. Choueiri at ASCO 2021 and subsequently published in NEJM.5 The trial design for KEYNOTE-564 is as follows:
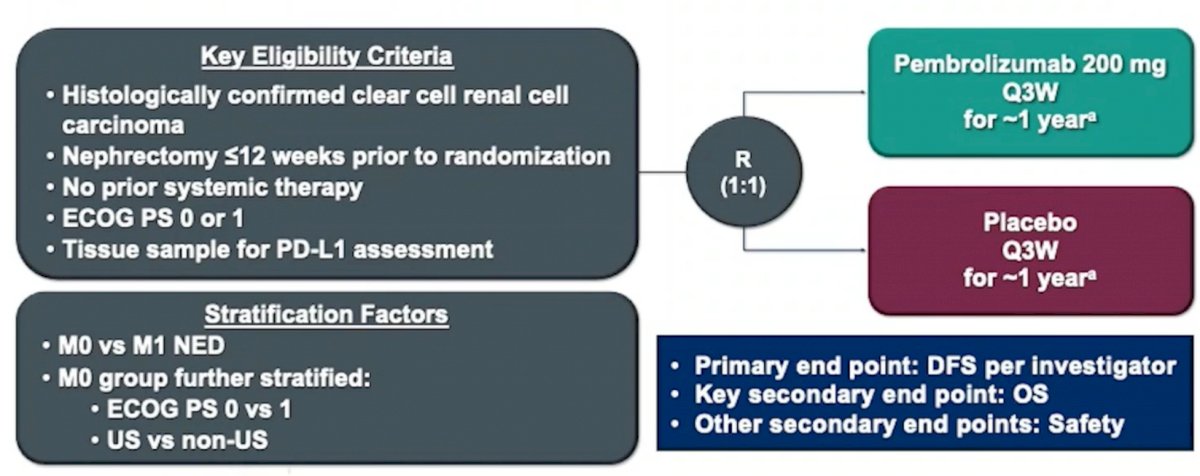
Dr. Bex notes that the inclusion criteria for this trial was intermediate-high risk disease (pT2 G4/sarcomatoid, pT3 Gany), high-risk disease (pT4 Gany, pTany GanyN1), and M1 resected to no evidence of disease (NED; <=1 year after surgery). At GU ASCO 2022, Dr. Choueiri presented 30-month follow-up from the KEYNOTE-564 trial, in which Dr. Bex notes that the DFS improvement was maintained (actually improved HR 0.68 HR 0.63) with subsequent follow-up:
Also with further follow-up, there does appear to be a survival benefit for adjuvant pembrolizumab:
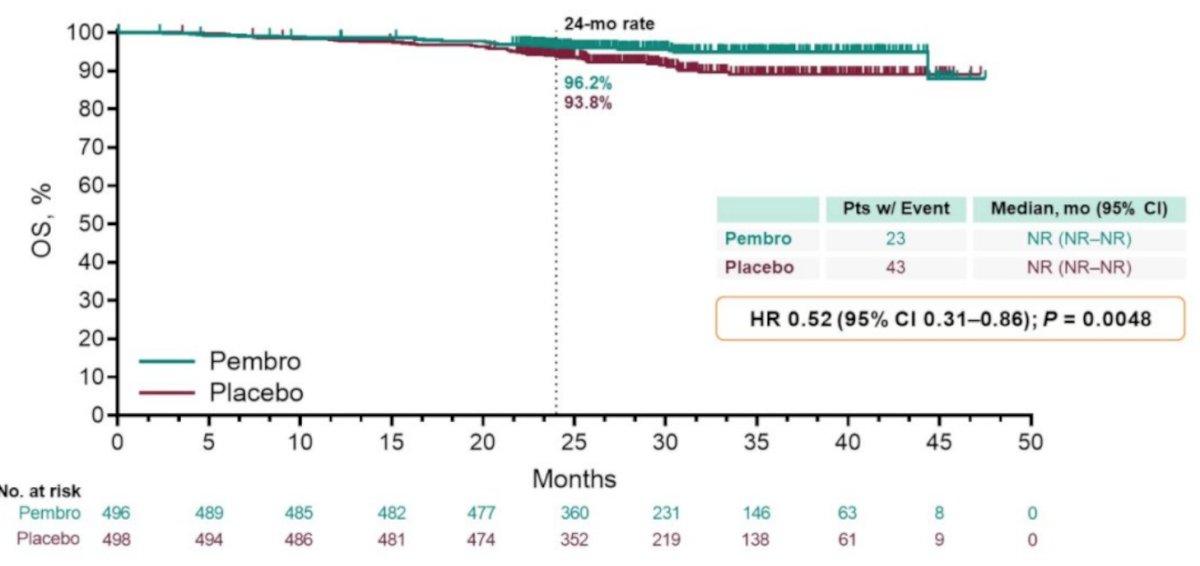
However, Dr. Bex cautions that the exploratory DFS hazard ratio for M1 NED (HR 0.29, 95% CI 0.12-0.69) is suggestive of treating systemic disease with systemic therapy and that perhaps this is what’s driving the survival difference (TKI-driven adjuvant trials after metastasectomy have shown no DFS or OS benefit). In KEYNOTE-564, patients in the M1 NED cohort had metastasectomy within 1 year after primary diagnosis. A metachronous interval of <1 year for recurrences following surgery with curative intent is a poor prognostic factor. Perhaps even more reason to not include M1 NED patients in adjuvant trials is that based on real-world data, surveillance of metastases is frequent (~32%) and a safe alternative to immediate systemic therapy with a median time to therapy of 16 months. So, Dr. Bex suggests that KEYNOTE-564 does not at this point answer the question of utility of metastasectomy, but perhaps the following hypothetical clinical trial design would:
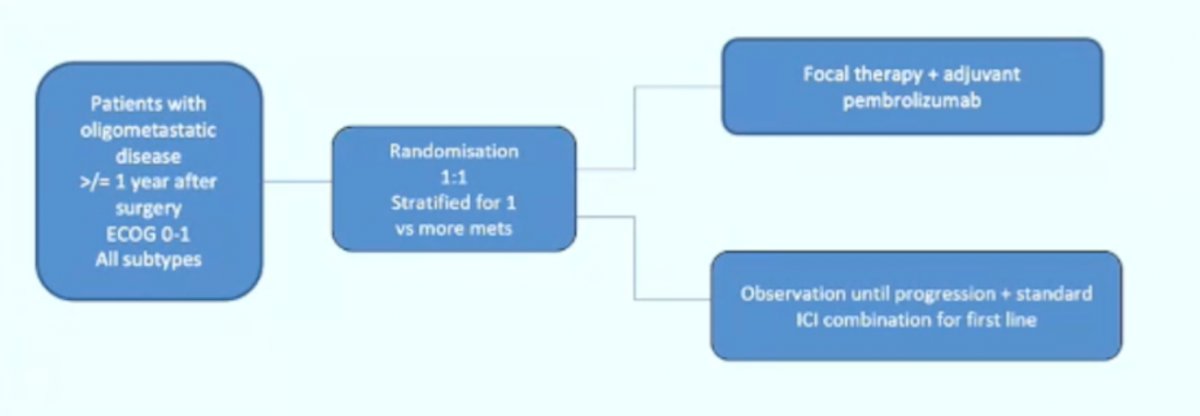
Dr. Bex concluded with several summary points regarding adjuvant immunotherapy that were recently added to the 2021 updated European Association of Urology Guidelines:6
- Immune checkpoint inhibitor therapy has a different mode of action than TKI resulting in complete response to 16% of patients with metastatic disease
- Despite immature OS data potentially driven by the M1 population, the panel cannot exclude that an OS benefit will emerge; this was not the case in the TKI trials
- Pembrolizumab is better tolerated than sunitinib and does not lead to a decline in quality of life
- We should perform a confirmatory axial scan of disease status prior to metastasectomy to rule out rapid progressive metastatic disease that requires systemic therapy
Presented by: Axel Bex, MD, PhD, Consultant Clinical Lead Specialist Centre for Kidney Cancer, The Royal Free London NHS Foundation Trust, Associate Professor UCL Division of Surgical and Interventional Science, London, United Kingdom
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 International Kidney Cancer Symposium (IKCS) Europe Annual Hybrid Meeting, Antwerp, Belgium, Fri, Apr 22 – Sun, Apr 24, 2022.
References:
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): A double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 2016;387(10032):2008-2016.
- Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N Engl J Med 2016;375(23):2246-2254.
- Motzer RJ, Haas NB, Donskov F, et al. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with locally advanced renal cell carcinoma (RCC) (PROTECT). J Clin Oncol 2017;35(35):3916-3923.
- Marconi L, Sun M, Beisland C, et al. Prevalence, Diseaes-free, and Overall Survival of Contemporary Patients with Renal Cell Carcinoma Eligible for Adjuvant Checkpoint Inhibitor Trials. Clin Genitourin Cancer. 2021;19(2):e92-e99.
- Choueiri TK, Tomczak P, Park SH, et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N Engl J Med. 2021 Aug 19;385(8):683-694.
- Bedke J, Albiges L, Capitanio U, et al. 2021 Updated European Association of Urology Guidelines on the Use of Adjuvant Pembrolizumab for Renal Cell Carcinoma. Eur Urol. 2022;81:134-137.


