(UroToday.com) In the fifteenth and final session of the 2022 International Kidney Cancer Symposium (IKCS): Europe meeting focusing on questions of therapeutic management dilemmas, Dr. Janet Brown presented working on optimizing treatment schedules with tyrosine kinase inhibitor (TKI) therapy.
She began by noting that TKIs dramatically changed the landscape of the treatment of locally advanced and metastatic renal cell carcinoma (mRCC) with significant demonstrated survival advantages compared to previously utilized treatments. While immune checkpoint inhibitor based therapies have recently become standard, TKIs are still used in the first-line setting and are the most commonly used second-line agents. However, these agents are associated with significant toxicity and 30-40% of patients require dose reduction or interruption. Therefore, approaches which reduce dosing intensity to reduce toxicity without compromising efficacy would be beneficial.
She highlighted first a non-randomized study of intermittent sunitinib therapy. All patients received an initial 4 cycle induction period and, if there was a 10% or greater reduction in tumor burden at the end of this period, patients were eligible for an intermittent phase. Among the roughly 60% of enrolled patients who were eligible for intermittent therapy, some were able to have prolonged breaks off-therapy (ranging from 3 to 44 months), while the majority had a saw tooth pattern with increasing tumor burden while off therapy and subsequent response and regression when treatment was resumed.
An alternative approach is to change to dosing schedule of sunitinib from the standard 4-weeks on / 2-weeks off cycle to a 2-weeks on / 1-week off regime. This has been evaluated in a number of small studies which have been summarized in a recent meta-analysis demonstrating that progression-free survival, overall survival, and stable disease rates were all significantly improved on this 2/1 schedule. Further, tolerability was increased with lower rates of fatigue, hypertension and diarrhea.
These early studies set the ground work for the STAR trial, a randomized phase II/III comparison of temporary cessation of standard first-line TKI compared with continuous treatment that Dr. Brown led. This study was based on the idea that intermittent therapy with a drug-free interval strategy (DFIS) may reduce toxicity and cost while preserving efficacy.
The trial schema is highlighted in the figure below and randomized patients were followed for the co-primary endpoints of overall survival and quality-adjusted life years, using a non-inferiority design.
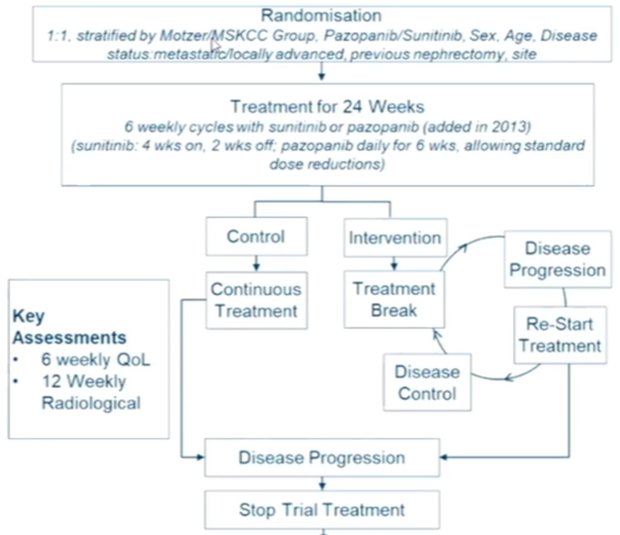
60 centers in the UK were involved in this trial and recruited 920 participants between January 2012 and September 2017. While randomized, this trial used a pragmatic design with as near as possible a real world population of patients, reflecting general oncologic practice in the UK at the time of recruitment.
While one break was mandated in the intervention arm, repeated breaks were allowed. The majority of patients ended up having one break (51%), with 15% having two and 27% having three or more. The median length of treatment break was 87 days (interquartile range 84 to 119). Despite these breaks, a similar number of treatment cycles was administered to patients in each arm.
Addressing their primary outcomes, the author found no difference in overall survival with a hazard ration of 0.97 (95% CI 0.83-1.12) in the intention to treat population and 0.94 (95% CI 0.80-1.09) in the per-protocol population. While the lower bound of the 95% CI is compatible with non-inferiority in the ITT population, it narrowly missed this cut off (0.812) in the PP population.
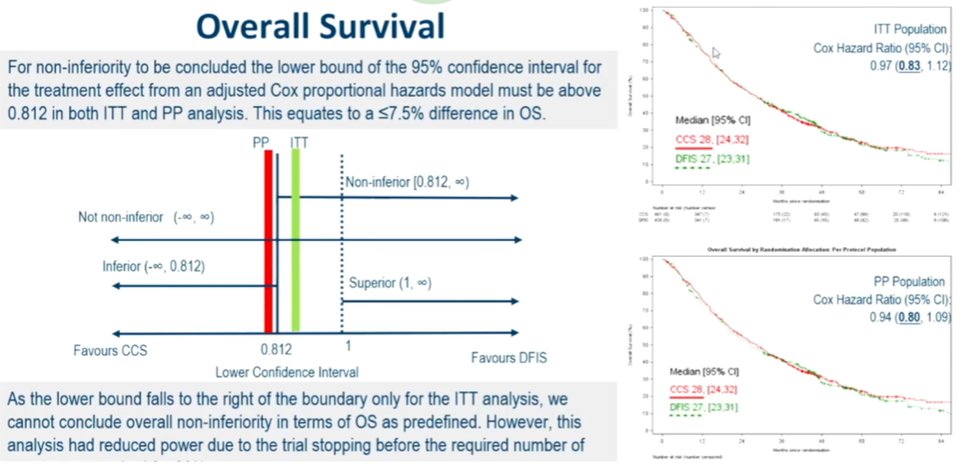
Subgroup analysis shows a general consistency of effect across strata, including those defined by risk groups. Looking next a quality-adjusted life years, the two treatment approaches were clearly non-inferior to each other. Finally, in terms of cost-effectiveness, the interrupted strategy was associated with significant cost-saving, driven primarily by reduced treatment costs.
In terms of secondary outcomes, Dr. Brown further highlighted that the interrupted treatment arm was associated with improvements in time to strategy failure and summative progression-free interval, as well as fewer severe adverse events.
While this approach was demonstrated to be acceptable to both patients and clinicians with potential benefits to the health care system, there is ongoing work to identify patients who benefit most. Further, this approach may be relevant for patients receiving immunotherapy and TKI based combination therapy in the first-line setting.


