The primary analysis of IMvigor130 was published this spring, demonstrating improved progression-free survival for patients who received atezolizumab plus platinum-based chemotherapy, as compared to platinum-based chemotherapy alone, in the first-line treatment of patients with advanced urothelial cancer.
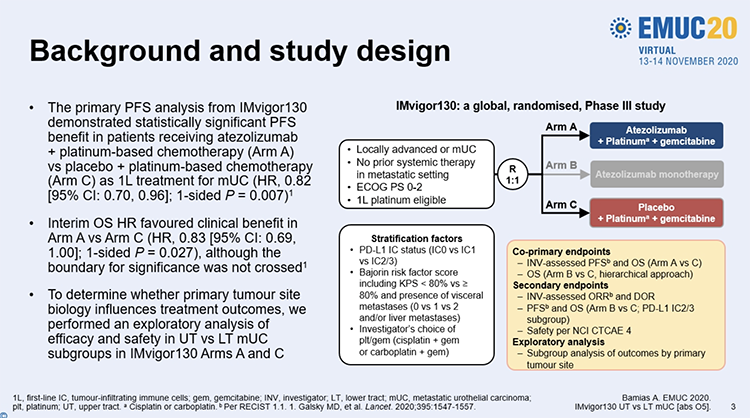
In the analysis presented by Dr. Bamias, they examined patients randomized to the atezolizumab and chemotherapy (Arm A) versus chemotherapy alone (Arm C). Patients randomized to atezolizumab monotherapy were not examined in this analysis. Based on eligibility for cisplatin, chemotherapy was given with gemcitabine and either cisplatin or carboplatin. The authors examined a number of endpoints including co-primary endpoints of progression-free survival (per RECIST 1.1) and overall survival.
The authors included 223 patients who had upper tract disease (renal pelvis/ureter) and 620 who had lower tract disease (bladder/urethra). Baseline characteristics were generally similar though, among patients with upper tract disease, there were proportionally more with PD-L1 IC2/3 status and proportionally fewer with Bajorin risk score of 2, among those randomized to atezolizumab and chemotherapy.
The authors found a survival benefit to the use of atezolizumab with chemotherapy, compared to chemotherapy alone, both among patients with upper tract disease (median survival benefit 2 months; hazard ratio 0.69, 95% confidence interval 0.51 to 0.94) and among those with lower tract disease (median survival benefit 1.6 months; hazard ratio 0.85, 95% confidence interval 0.70 to 1.02).

Interim analysis of overall survival similarly demonstrated benefit in both subgroups with a median overall survival benefit of 3.4 months (hazard ratio 0.78, 95% confidence interval 0.54 to1.12) among those with upper tract disease and a median survival benefit of 2.4 months (hazard ratio 0.87, 95% confidence interval 0.70 to 1.08).
Toxicity was similar between patients with upper tract disease and those with lower tract disease.
The authors conclude that these data support the role of atezolizumab with chemotherapy among patients with both upper tract and lower tract urothelial carcinoma, though ongoing analyses of overall survival will be conducted.
Presented by: Aristotelis Bamias, MD, University of Athens, Greece
Written by: Christopher J.D. Wallis, MD, Ph.D., Urologic Oncology Fellow, Vanderbilt University Medical Center Contact: @WallisCJD at the 12th European Multidisciplinary Congress on Urological Cancers (EMUC) (#EMUC20 ), November 13th - 14th, 2020
Related Content:
ESMO Virtual Congress 2020: Patient-Reported Outcomes from IMvigor130: Atezolizumab + Platinum-Based Chemotherapy vs placebo + PBC in Previously Untreated Locally Advanced or Metastatic Urothelial Carcinoma


