Dr. Attard notes that evidence suggests that 90% of metastatic castration-resistant prostate cancer (mCRPC) have actionable genomic aberrations and that there is evidence to suggest that homologous recombination repair pathway (HRR) mutations are truncal and present at diagnosis:
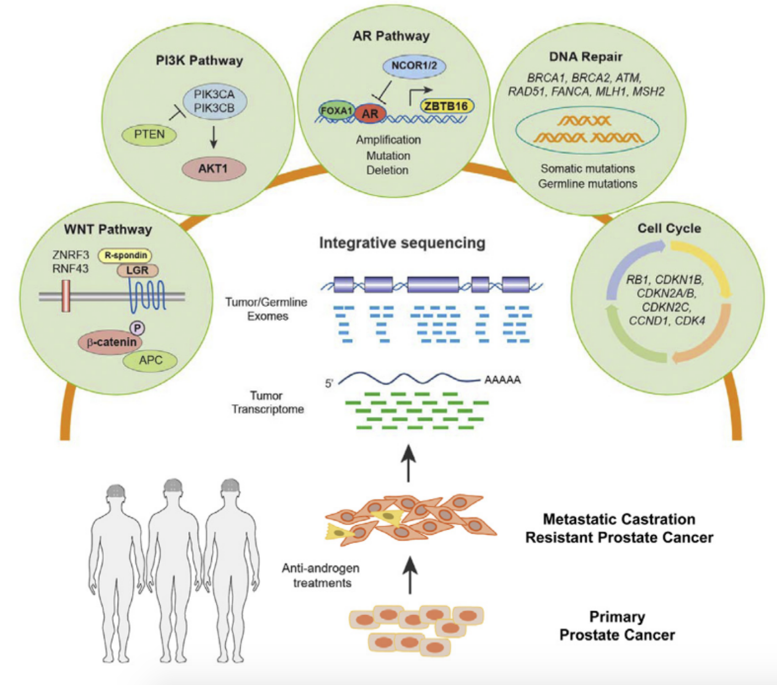
Based on seminal work from Dr. Pritchard and colleagues, 6-11% of lethal CPRC have germline DNA repair gene defects, with the majority of germline carriers having had a 2nd somatic hit.1 Furthermore, BRCA2 germline variants have worse outcomes from mCRPC onset; this is even more abundant in the absence of poly ADP ribose polymerase (PARP) inhibitors or platinum chemotherapy. The PROfound trial recently published overall survival data in the New England Journal of Medicine,2 noting that the median duration of overall survival in cohort A was 19.1 months with olaparib and 14.7 months with control therapy (HR 0.69, 95% CI 0.50 to 0.97; p = 0.02). In cohort B, the median duration of overall survival was 14.1 months with olaparib and 11.5 months with control therapy, and finally, in the overall population (cohorts A and B), the corresponding durations were 17.3 months and 14.0 months. Dr. Attard highlighted that exploratory gene-level analyses showed hazard ratios for death (olaparib vs. control) of 0.42 (95% CI, 0.12 to 1.53) among patients with an alteration in only BRCA1 and 0.59 (95% CI, 0.37 to 0.95) among patients with an alteration only in BRCA2:

Additionally, approximately 3% of metastatic prostate cancer patients have microsatellite instability-high/mismatch repair-deficient disease, opening the door for immune checkpoint blockade for these patients.
With regards to which test to use, Dr. Attard notes that plasma DNA is used for selecting mCRPC patients for poly ADP ribose polymerase (PARP) inhibitors. Recently, the United States (US) Federal Drug Administration (FDA) approved FoundationOne® Liquid CDX to serve as a rucaparib companion diagnostic for identifying eligible patients with BRCA1/2-mutant mCRPC. When to test patients (how early in the prostate cancer disease spectrum) has been a point of much debate, but certainly, all patients that are considering a treatment change or those that may be a candidate for olaparib/pembrolizumab should be tested.
Dr. Attard concluded his presentation by noting several challenges in using tumor mutations to influence patient care:
- There is significant intra-patient tumor heterogeneity
- Interpreting anomalies of unknown function
- Access to actionable therapeutics
- Cost, infrastructure and timelines
- Tumor evolution and rapid emergence of resistant clones
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia at the 12th European Multidisciplinary Congress on Urological Cancers (EMUC) (#EMUC20 ), November 13th - 14th, 2020
References:
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453.
- Hussain M, Mateo J, Fizazi K, et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020 Sep 20 [Epub ahead of print].
The Role of FoundationOne® Liquid CDx in mCRPC - Oliver Sartor


