There was a lot of retrospective data (particularly in Australia) with PSMA PET-CT, Dr. Murphy and colleagues set out to assess prospectively the use of PSMA PET-CT among patients with high-risk prostate cancer before curative-intent surgery or radiotherapy – the proPSMA study,1 A prospective randomised multi-centre study of the impact of Ga-68 PSMA-PET/CT imaging for staging high-risk prostate cancer prior to curative-intent surgery (proPSMA). To be eligible for inclusion in this trial, men must have had at least one high-risk factor including PSA >= 20 ng/mL, ISUP grade group 3-5, or clinical stage T3 or greater. Patients who had undergoing staging investigations (apart from prostate MRI) within eight weeks prior to randomization were excluded. Following enrollment, patients were randomly assigned in a 1:1 ratio to either conventional imaging performed using bone scan and CT or PSMA PET/CT. Patients who were randomized to conventional imaging underwent an abdominopelvic CT scan with contrast as well as a technetium-99m bone scan with SPECT CT of chest, abdomen, and pelvic in keeping with the standard of care. For patients randomized to PET/CT, gallium-68 PSMA-11 PET/CT was performed. In patients who had fewer than three unequivocal sites of metastasis, cross-over imaging for confirmation was performed within 14 days. Confirmatory testing following imaging was performed at the discretion of the treating physician and included biopsy confirmation. The study schema is as follows:
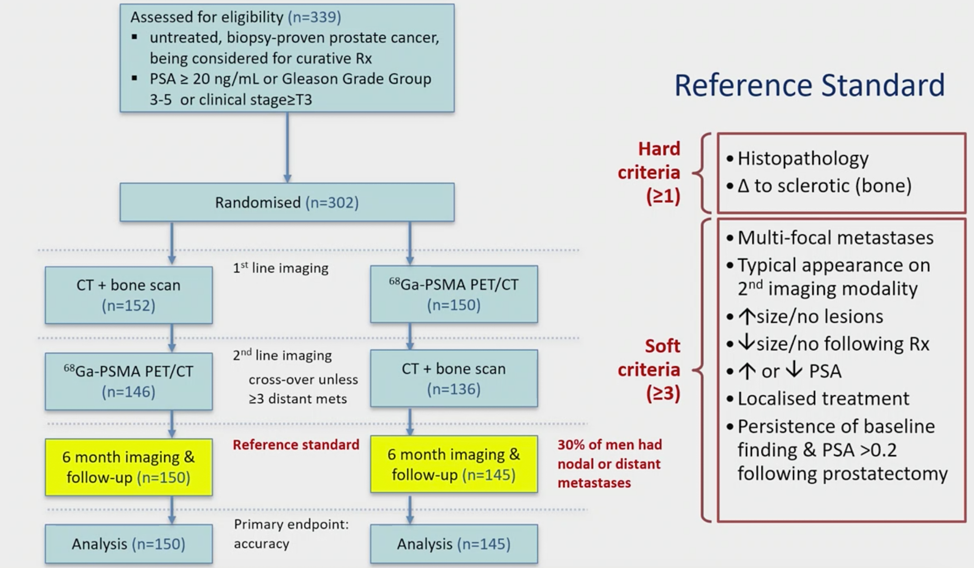
The primary study outcome was the accuracy of first-line diagnostic imaging for the identification of either pelvic nodal or distant metastatic disease. Accuracy was assessed using the area under the curve (AUC) of the receiver operating characteristic (ROC) curve. The reference standard was a composite panel of histopathology, imaging, clinical, and biochemical characteristics.
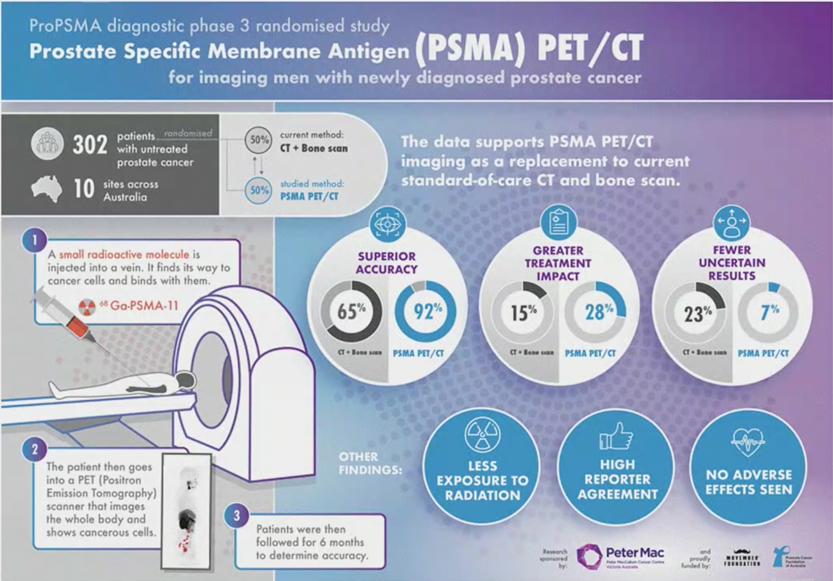
Between 2017 and 2018, the authors randomly assigned 302 patients of whom 300 received assigned first-line imaging. In the primary outcome assessment, PSMA PET-CT had a 27% absolute greater AUC for accuracy compared to conventional imaging (95% CI 23-31): 92% (95% CI 88-95%) vs. 65% (60-69%). Conventional imaging had both a lower sensitivity (38% vs. 85%) and also a lower specificity (91% vs. 98%).
Prior to treatment, the results of conventional imaging studies resulted in treatment change for 23 men (15%, 95% CI 10-22) while the results of PSMA PET-CT resulted in treatment change for 41 (28%, 95% confidence interval 21-36). These changes included both a transition from curative intent to palliative intent treatment in 20 patients (14%) and also a change in treatment approach in 22 (14%). Additionally, conventional imaging was associated with a higher radiation dose (19.2 mSv compared to 8.4 mSv; absolute difference 10.9 mSv, 95% CI 9.8-12.0 mSv0. PSMA PET-CT was not associated with any adverse events and reporter agreement was high for both nodal (kappa 0.87, 95% CI 0.81-0.94) and distant metastatic disease (kappa 0.88, 95% CI 0.94-0.92).
The strengths of the proPSMA study are the multi-center, randomized approach, providing real-world data. Furthermore, it allowed a direct comparison to CT and bone scan, both as a replacement or as second-line imaging. Weaknesses of the study include the inability (at least with current follow-up) to assess whether these changes will improve outcomes in these patients. Furthermore, as has been the issue with PSMA PET-CT from the outset, it has limited availability, and cost-effectiveness studies are still needed. As follows is a Visual Abstract summary of the proPSMA study:
According to Dr. Murphy, there are several important considerations on management impact based on the results of proPSMA:
- If conventional imaging is negative, how much should we abandon the standard of care? (ie. surgery or radiotherapy)
- Should we try harder for tissue diagnosis?
- How should we assess the management impact? Hard endpoints in prostate cancer take >10 years to occur
The most recent European Association of Urology (EAU) guidelines regarding the initial staging of disease state that “results from randomized control trials (RCTs) evaluating the management and outcome of patients with (and without) metastases detected by choline PET/CT, PSMA PET/CT, and MRI are awaited before a decision can be made to treat patients based on the results of these tests”. Dr. Murphy notes that novel imaging is more sensitive, but the benefit of detecting metastases earlier is unclear. Also, it is still unclear if PET-only metastases should be treated systematically, or aggressive local and metastasis-directed therapy.
Dr. Murphy concluded with the following take-home messages for PSMA PET/CT for primary staging:
- proPSMA has established superior accuracy of PSMA PET/CT for primary staging
- It is associated with less radiation, less equivocal findings, and leads to a significant change in management
- The cost-effectiveness data is currently in press for publication
- FDA approval is imminent
- The management impact needs to be further evaluated, but in Dr. Murphy’s opinion PSMA PET/CT should replace conventional imaging for high-risk and unfavorable intermediate-risk prostate cancer
Presented by: Declan Murphy, MB, BCH, BaO, FRACS, FRCS, Urol, Peter MacCallum Cancer Centre, Melbourne, Australia
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 12th European Multidisciplinary Congress on Urological Cancers (EMUC) (#EMUC20 ), November 13th - 14th, 2020
References:
Related Content:
PSMA PET/CT Imaging for Staging High-risk Prostate Cancer Prior to Curative-intent Surgery or Radiotherapy (proPSMA) - Michael Hofman and Declan Murphy


