
CheckMate 275 was a phase II, single-arm study where patients received nivolumab 3 mg/kg intravenously every 2 weeks until disease progression and clinical deterioration and confirmed objective responses were achieved in 52 (19.6%, 95% CI 15.0–24.9) of 265 patients. There were six patients (2%) who achieved a complete response and 46 (17%) who achieved a partial response. Median overall survival was 8.74 months (95% CI 6.05 to not reached) in the overall population. KEYNOTE-045 was an open-label, international, randomized phase 3 trial, comparing pembrolizumab to investigator’s choice of chemotherapy with paclitaxel, docetaxel, or vinflunine. This study also showed an overall survival benefit – patients in the pembrolizumab arm had a median OS of 10.3 months (95% confidence interval [CI], 8.0 to 11.8) compared with 7.4 months (95% CI, 6.1 to 8.3) in the chemotherapy group (HR, 0.73; 95% CI, 0.59 to 0.91; P=0.002).
What is unknown at this time is if the combination checkpoint blockade ipilimumab with nivolumab is superior to single-agent PD-1/PD-L1 blockade. This combination has been successful with improving overall survival in selected patients with metastatic melanoma (CheckMate 0676) and advanced renal cell carcinoma (CheckMate 2147). This abstract adds to the literature on the efficacy of combined CTLA-4 and PD-1 blockade for UC.
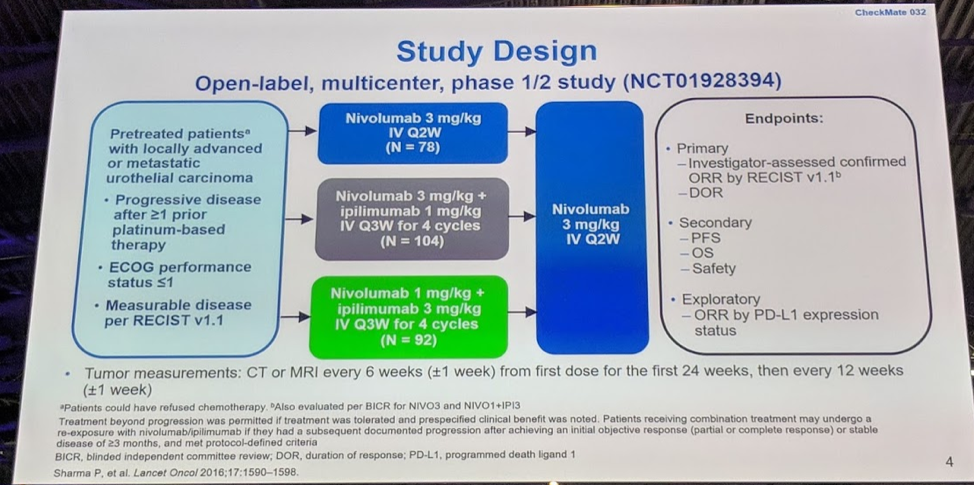
In this study, pre-treated patients with metastatic UC were treated with one of three regimens: nivolumab 3 mg/kg every 2 weeks (Nivo3), nivolumab 3 mg/kg + ipilimumab 1 every 3 weeks for 4 cycles (Nivo3Ipi1), and nivolumab 1 mg/kg + ipilimumab 3 mg/kg every 3 weeks for 4 cycles (Nivo1Ipi3). All three cohorts were given nivolumab 3 mg/kg every 2 weeks after the initiation “induction” phase.
There were 76 patients in Nivo3, 104 patients in Nivo3Ipi1, and 92 patients in Nivo1Ipi3. Approximately 1/3 of patients had liver metastases and half of the patients had a PD-L1 expression of <1%. Most patients were accrued from the United States for Nivo3 and Nivo3Ipi1, and the reverse was seen for Nivo1Ipi3, with the majority (66%) recruited from the rest of the world.
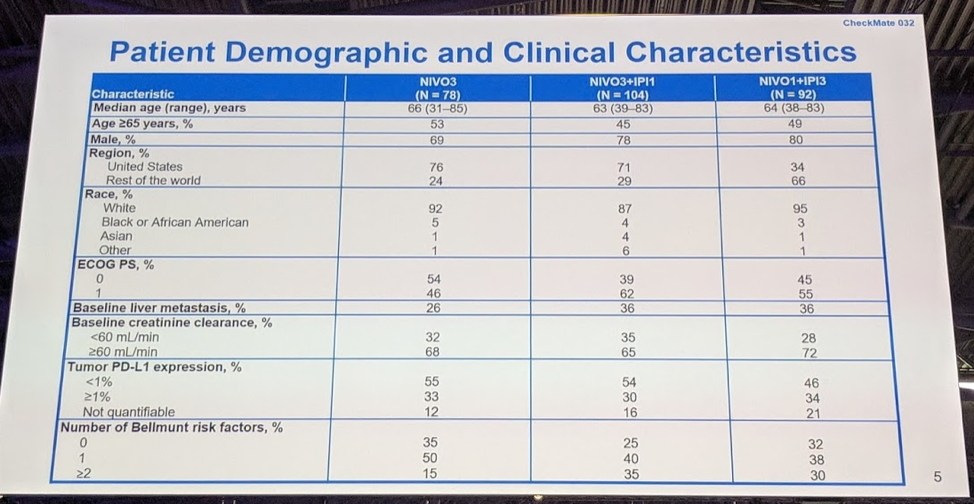
In terms of prior treatment, this was a heavily pre-treated population - 44-54% of patients had 2-3 prior treatment regimens, and 75-85% of patients had prior systemic therapy for metastatic disease.

At the time of this reporting, a minimum of 37.7 months of reporting have been completed for the first two arms (Nivo3 and Nivo3Ipi1), and the minimum follow up for Nivo1Ipi3 is still immature at 7.9 months. 8-9% of patients are still on therapy for Nivo3 and Nivo3Ipi1, at this current time, 24% of patients are still on therapy for Nivo1Ipi3. Most patients who have discontinued therapy did so due to disease progression. Discontinuation due to study drug toxicity was most common in the combination arms (13-14%) compared with the Nivo3 arm (4%).

In terms of response, the confirmed overall response was greatest in the Nivo1Ipi3 arm (38%), compared with Nivo3 (25.6%) and Nivo3Ipi1 (26.9%). At this time, Nivo3 has the highest complete response rate (10.3%), compared to Nivo3Ipi1 (7.7%) and Nivo1Ipi3 (6.5%). However, as the author and discussant mentioned, the data for Nivo1Ipi3 is still immature and the rate of CRs may increase over time.

The response to immune checkpoint inhibition occurred rapidly, with a median time to response of only 1.4-2.0 months across the three cohorts and amongst the responders, the response was quite durable. For Nivo3, the median duration was 30.5 months, with some patients now with over 44 months of response. The median duration was 22.3 months in the Nivo3Ipi1 arm and 22.9 months in the Nivo1Ipi3 arm.
Waterfall plots show that the median tumor reduction from baseline shows an impressive 30% in the Nivo1Ipi3 arm, which also had the longest median progression-free survival at 4.9 months, compared to 2.6 months for Nivo3Ipi1 and 2.8 months for Nivo3.


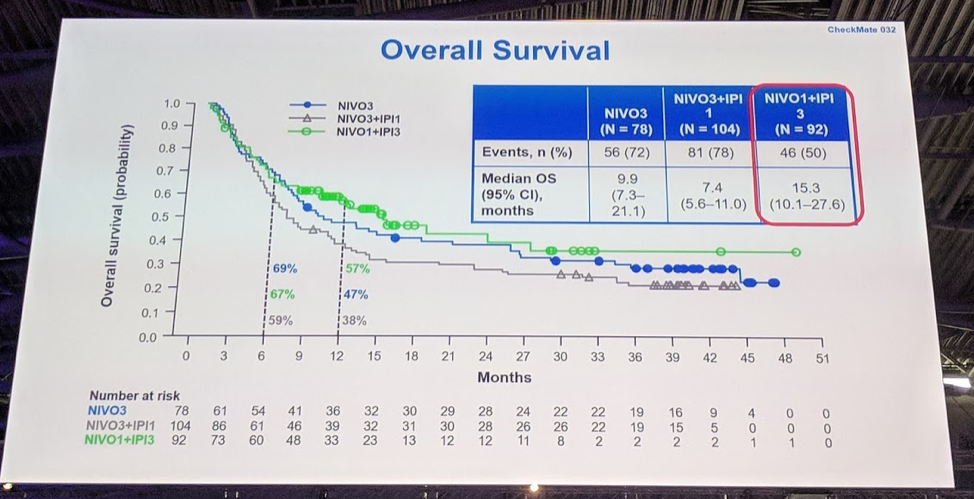
Overall survival data showed an impressive median OS of 15.3 months for Nivo1Ipi3 at this time, and all three cohorts appear to have a durable tail although data for Nivo1Ipi3 is still immature.
Correlative studies evaluating tumor PD-L1 showed that the majority of patients who had a PD-L1>1% had a response with Nivo1Ipi3 (58.1%) and this was confirmed by the blinded independent central review (54.8%).

In terms of tolerability, the typical immune-related adverse events were reported including diarrhea and rash. More patients who received ipilimumab had diarrhea and hepatitis than patients who were on the nivolumab alone arm.
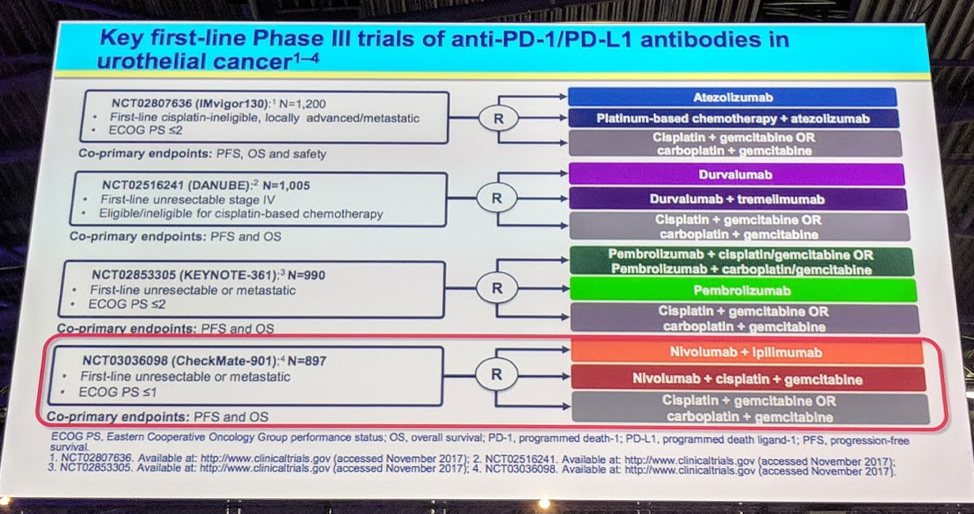
This study demonstrates that the combination of nivolumab and ipilimumab is tolerable and has good efficacy for patients with pretreated metastatic UC. The discussant does note that this study was not a phase III randomized controlled trial, but rather a phase I/II study, and thus we are unable to compare efficacy between the three arms, and unable to compare this study results with other phase III trials in this patient demographic. However, these results are exciting for our patients with metastatic UC and suggest that there exist a subset of patients who can have a very durable response to single agent and combination immune checkpoint inhibition. Future important studies include Checkmate 901, an ongoing phase III clinical trial which randomly assigns patients to chemotherapy, Ipi/Nivo, and Nivolumab + chemotherapy, and this trial will offer important insights in the treatment of our patients with metastatic urothelial carcinoma.
Presented by: Jonathan E. Rosenberg, Memorial Sloane Kettering Cancer Center, New York, US
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, Twitter: @TheRealJasonZhu at the 2018 European Society for Medical Oncology Congress (#ESMO18), October 19-23, 2018, Munich Germany
References:
1. Bellmunt J, De Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. New England Journal of Medicine 2017;376:1015-26.
2. Powles T, Loriot Y, Duran I. IMvigor211: a phase III randomized study examining atezolizumab vs. chemotherapy for platinum-treated advanced urothelial carcinoma. 2nd Special Conference EACR AACR SIC; 2017.
3. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. The Lancet Oncology 2017;18:312-22.
4. Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an anti–programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase ib study. Journal of Clinical Oncology 2017:JCO. 2016.71. 6795.
5. Powles T, O'Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study. JAMA Oncology 2017;3:e172411.
6. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. New England Journal of Medicine 2017;377:1345-56.
7. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. New England Journal of Medicine 2018;378:1277-90.


