Munich, Germany (UroToday.com) Cora Sternberg, MD gave an overview of the contemporary role of immunotherapy in renal and bladder cancer. Immunotherapy in renal cancer began with the introduction of high dose IL-2 therapy in a total of 7 clinical trials including 255 patients.1 IL-2 was FDA approved in 1992 demonstrating 15% risk reduction with durable responses in a small percentage of patients. However, this treatment caused significant toxicity and had considerable cost.
It was limited to selected patients treated at a few centers. Since the introduction of cytokines, targeted therapies have been developed and recently immunotherapy has been upgraded. Currently, there are 10 approved drugs that have been introduced in the last 10 years (figure 1).
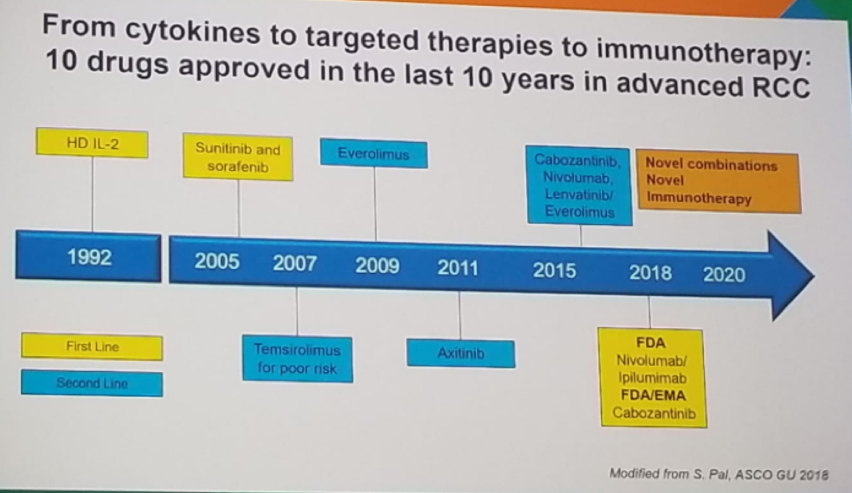
Figure 1
The Checkmate 025 is a phase 3 study with 821 advanced or metastatic clear cell patients, that randomized them to either everolimus or nivolumab.2 The primary endpoint was overall survival, and patients were treated until progression or intolerable toxicity. The results demonstrated an overall response rate of 25% vs. 5%, favoring nivolumab. No difference was seen in progression free survival, but a clear difference was seen in overall survival favoring nivolumab. Half of the patients had a reduction in tumor burden, and 14% had over 30% reduction in tumor burden.
There are several first line combination trials which are currently closed for accrual. The first is the Checkmate 214 comparing sunitinib to nivolumab and ipilumab. Additional trials are the phase 3 IMmotion 151 comparing sunitinib to atezolizumab and bevacizumab, and the ADAPT trial, comparing sunitinib to AGS-003 + sunitinib. There are other first line trials which are still ongoing, like the Keynote 426, comparing sunitinib to axitinib and pembrolizumab.
There is a preclinical and clinical rationale for combined PD-1 blockade (nivolumab) and CTLA-4 blockade (ipilimumab) in melanoma, non-small cell lung cancer, and metastatic renal cell carcinoma, (Figure 2).
Figure 2: Combined immunotherapy blockade:
When assessing the results of Checkmate 214, comparing sunitinib to nivolumab and ipilimumab, the complete response rate was 9% for the nivolumab arm, compared to 1% in the sunitinib arm. When looking at the progression free survival (PFS) and overall survival (OS), in the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) intermediate/poor risk patients, the nivolumab patients fared better with a hazard ratio of 0.82 (0.64-1.05), p=0.0331, and a hazards ratio of 0.63 (0.44-0.89), p<0.001, respectively. In contrast, in the favorable IMDC risk group, the sunitinib patients fared better with a hazard ratio of 2.18 (1.29-3.68), p<0.0001. When stratifying by the PD-L1 status, there was no difference in PFS between both arms in patients with PD-L1<1%, although the nivolumab patients fared much better in the patients with PD-L1 >=1%, with a hazards ratio of 0.48 (0.28-0.82), p=0.0003. A similar result was demonstrated with OS when stratified by PD-L1 expression.
In IMmotion 151 sunitinib was compared to atezolizumab and bevacizumab. The combination treatment was shown to be better than sunitinib in PFS, objective response rate, and OS. In keynote 426, comparing sunitinib to axitinib and pembrolizumab, more than 90% of patients had some tumor shrinkage in the axitinib group, with an objective response rate of 73.1%. The median time to response was 2 Months with a median duration of response is 18.6 months, and the median overall survival was not reached.
These trials have resulted in an update in the ESMO guidelines (Figure 3), where sunitinib is recommended for good risk patients, and nivolumab and ipilimumab recommended for intermediate and poor risk patients.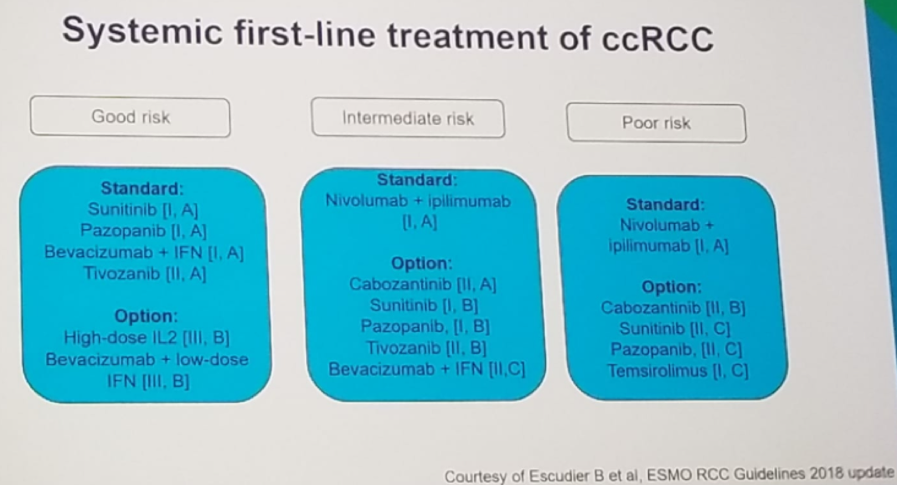
Figure 3- ESMO guidelines for Metastatic Renal Cell Cancer:
Dr. Sternberg moved on to discuss bladder cancer, describing the recent progress, compared to the last 30 years of no new drug developments (Figure 4). Bladder cancer is one of the tumors with the highest load of somatic mutations, making it a perfect target for immunotherapy. In the last few years, five new immunotherapeutic agents were developed - atezolizumab, durvalumab, avelumab, nivolumab, and pembrolizumab.
Immunotherapy in bladder cancer has been initially tested in two settings: prior platinum treatment, or cis-platinum ineligible patients. In the platinum-refractory setting, there has been no head to head comparisons, but initial data has shown an objective response rate of around 20%, a median OS of 7.7-18.2 months, and a median PFS of 1.5-2.1 months for all 5 immune check point inhibitors (Figure 5).
Using analysis of The Cancer Genome Atlas (TCGA), it was demonstrated that luminal subtype II had an objective response rate that was significantly higher than the other subtypes (p=0.0072), Figure 6.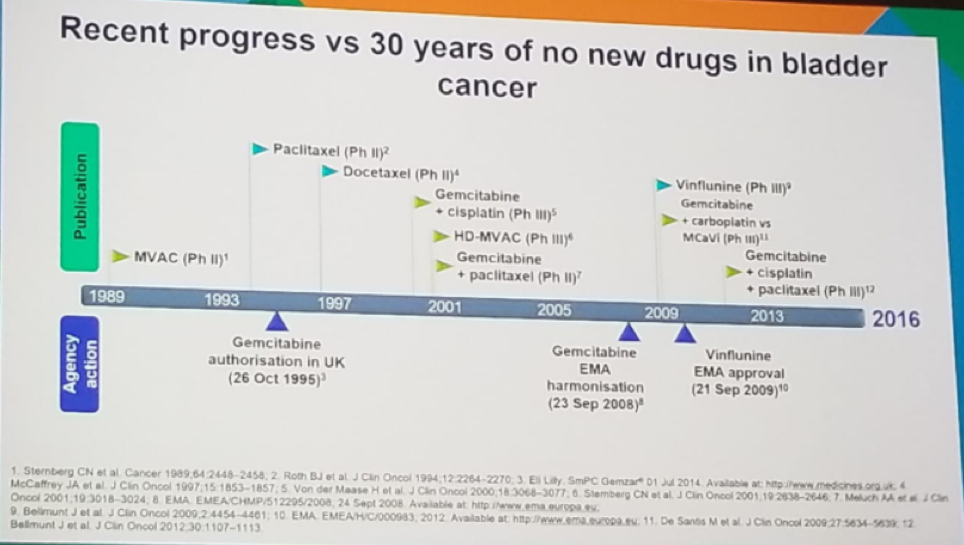
Figure 4 – Bladder cancer treatment in the last few decades: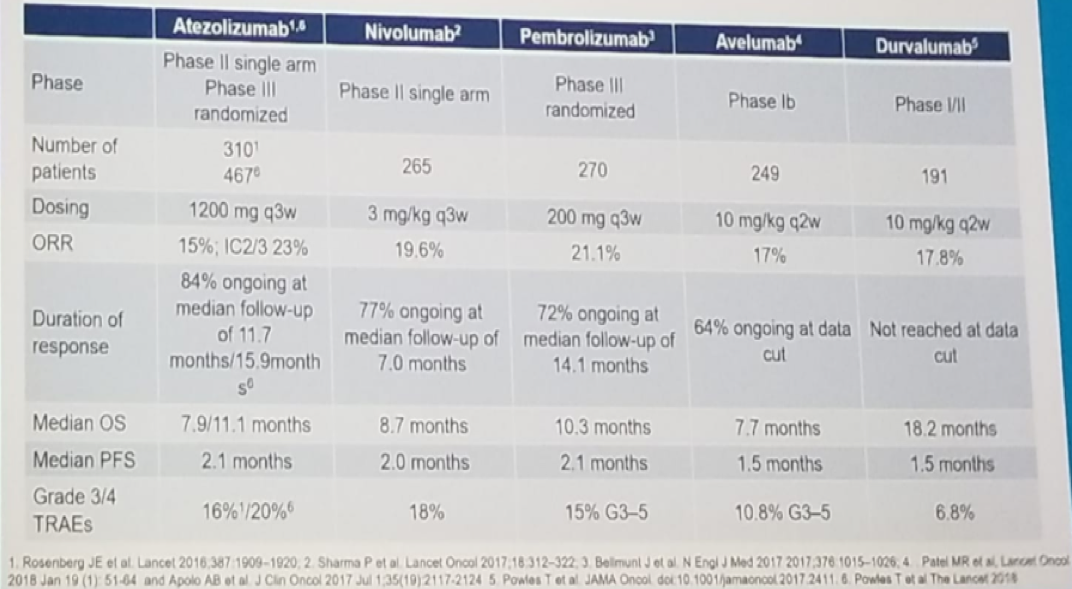
Figure 5: Immune checkpoint inhibitors in the platinum-refractory setting of bladder cancer: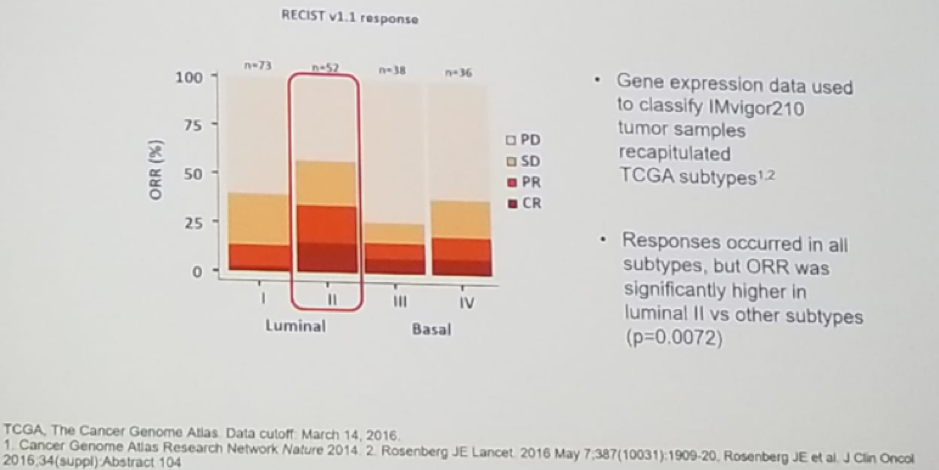
Figure 6 -TCGA subtype is associated with a higher objective response rate:
In the Checkmate 275 study, metastatic urothelial carcinoma of the bladder patients, who progressed on prior platinum-based therapy, were given nivolumab with the primary endpoint being objective response rate. This trial demonstrated that the objective response rate was 19.6% and median OS in all patients was 8.7 months.3
Another trial, the Keynote-045 phase 3 study, took similar patients, after 1-2 lines of cis-platinum therapy and randomized them to either pembrolizumab or paclitaxel for a duration of two years or until acceptable toxicity4. This trial showed a clear benefit in overall survival in favor of pembrolizumab of 43.9% vs. 30.7%.
Another discussed trial was the IMvigor 211 phase 3 study in platinum-refractory patients, compared chemotherapy (investigator’s choice) to pembrolizumab in a total of 931 patients, with the primary endpoint being OS.5 In the intention to treat analysis, the trial demonstrated an advantage to the atezolizumab arm with a median OS of 8.6 months. vs. 8 months, p=0.038. Similarly, durvalumab and avelumab also demonstrated an advantage in pretreated metastatic urothelial cancer.6,7
The last topic discussed was the use of immune checkpoint inhibitors as first-line treatment in cisplatin-ineligible patients (Figure 7), demonstrating an objective response rate of 23-29%, and a median OS f 11.5-15.9 months. Many more studies exist on the first line phase 3 trials of anti-PD1/PD-L1 antibodies in urothelial cancer (Figure 8).
Figure 7: Immune checkpoint inhibitors in first line cisplatin-ineligible patients: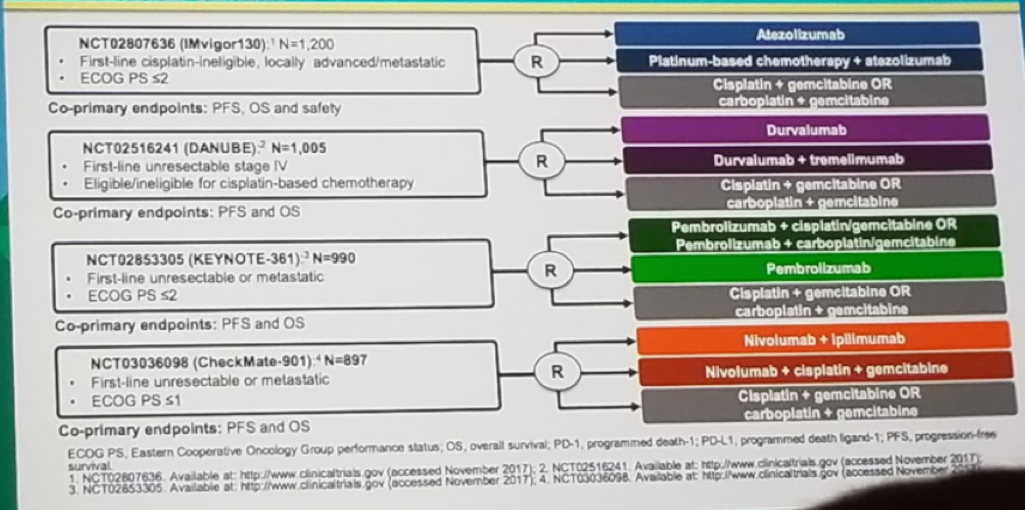
Figure 8 – First line phase 3 trials of anti-PD1/PD-L1 antibodies in urothelial cancer:
Dr. Sternberg continued and summarized that first-line therapy for metastatic urothelial carcinoma still includes chemotherapy and that patients that don’t have any issues receiving cisplatin, should get it. If the PD-L1 count is low, the FDA and the European Medicine Agency (EMA) warn of decreased survival in cis-platin ineligible patients receiving first-line therapy with immune checkpoint inhibitors.
There are additional studies underway assessing the role of immune-checkpoint inhibitors as a neoadjuvant treatment before radical cystectomy, adjuvant treatment, and as maintenance therapy.
Dr. Sternberg concluded her talk stating that immunotherapy has arrived for urothelial cancer. This cancer has a high mutational load, with the potential for many neoantigens that could trigger an immune response. PD-L1 expression does not have a clear role in urothelial cancer treatment. It is important that the adverse events managed properly, and that combination therapy may be of interest.
Presented by: Cora Sternberg, MD, FACP, Chief of the Department of Medical Oncology, San Camillo-Forlanini Hospital and Adjunct Professor, La Sapienza University, Rome, Italy, Adjunct Professor, Tufts University School of Medicine and the Lahey Clinic, Boston, Massachusetts
References:
1. Fyfe GA et al. JCO 1995
2. Motzer RJ et al. NEJM 2015
3. Sharma P et al. Lancet Oncol 2017
4. Bellmunt J et al. NEJM 2017
5. Poweles T et al. Lancet 2017
6. Poweles T et al. Jama Oncol 2017
7. Patel MR et al. Lancet Oncol 2018
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 2018 European Society for Medical Oncology Congress (#ESMO18), October 19-23, 2018, Munich Germany


