Munich, Germany (UroToday.com) Cabazitaxel, abiraterone, and enzalutamide have demonstrated benefit in randomized controlled trials for patients with mCRPC. Abiraterone was shown to be effective in patients pre and post-chemotherapy in Cougar 301 and Cougar 3021,2. In patients who had not been treated with docetaxel, abiraterone significantly improved median overall survival compared with placebo (34.7 months vs 30.3 months, HR 0.81 (95% CI 0.70–0.93), p=0·0033). In patients who had progressed on docetaxel, overall survival with abiraterone was 14.8 months, compared with 10.9 months on placebo (HR 0.65, 95% CI 0.54 to 0.77; P<0.001).
Enzalutamide has also been shown to be effective in the pre-chemotherapy and post-chemotherapy stage3,4. Prior to chemotherapy, radiographic progression-free survival at 12 months was 65% among patients treated with enzalutamide, compared with 14% among patients who received placebo4. Post-chemotherapy, median overall survival was 18.4 months in the enzalutamide group versus 13.6 months in the placebo group (HR 0.63; 95% CI, 0.53 to 0.75; P<0.001)3.
TROPIC was the phase 3 trial that established a role for cabazitaxel after patients progressed on docetaxel treatment. In TROPIC, patients were given prednisone daily and randomly assigned to either 12 mg/m2 of mitoxantrone or 25 mg/m2 cabazitaxel every 3 weeks. The median survival was 15.1 months (95% CI 14.1–16.3) in the cabazitaxel group and 12.7 months (95% CI 11.6–13.7) in the mitoxantrone group, with a hazard ratio of 0.70 (95% CI 0.59–0.83, p<0.0001).
These three therapies described above all have strong evidence of efficacy in mCRPC, but there is insufficient data on how to best sequence these therapies. A phase II study compared the sequencing and enzalutamide and abiraterone but they did not include cabazitaxel5. In that study, 202 patients were randomized to starting abiraterone or enzalutamide, with cross-over to the other therapy at the time of PSA progression. In terms of a PSA ≥50% decline (PSA50) from baseline, more patients achieved this with enzalutamide than abiraterone (73% vs 75%, p=0.004). However, there was no difference between starting abiraterone or enzalutamide in terms of median time to PSA progression 7.4 vs 8.0 months (HR = 0.88, 95% CI 0.61, 1.27).
This study randomized 95 patients to either first-line therapy with cabazitaxel or an androgen antagonist (enzalutamide or abiraterone). This was a high-risk population of patients with multiple poor prognostic factors required, including liver metastases, CRPC within 12 months of ADT initiation for metastatic disease, and the presence of at least 4 high-risk features including LDH> upper limit of normal (ULN), ECOG PS 2, albumin <4, Alkaline phosphatase >ULN, and <36 months from the time of ADT. Accrual was stopped early due to slow enrollment. 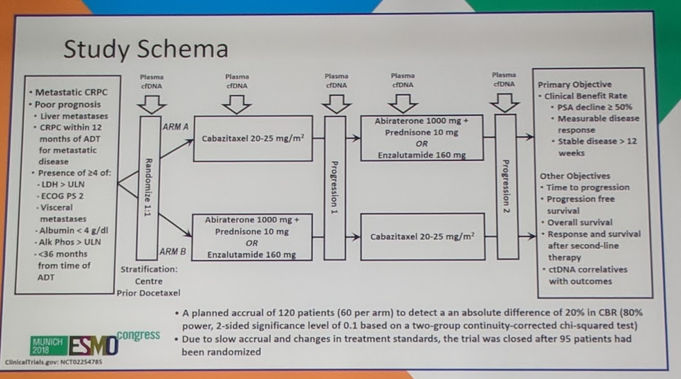
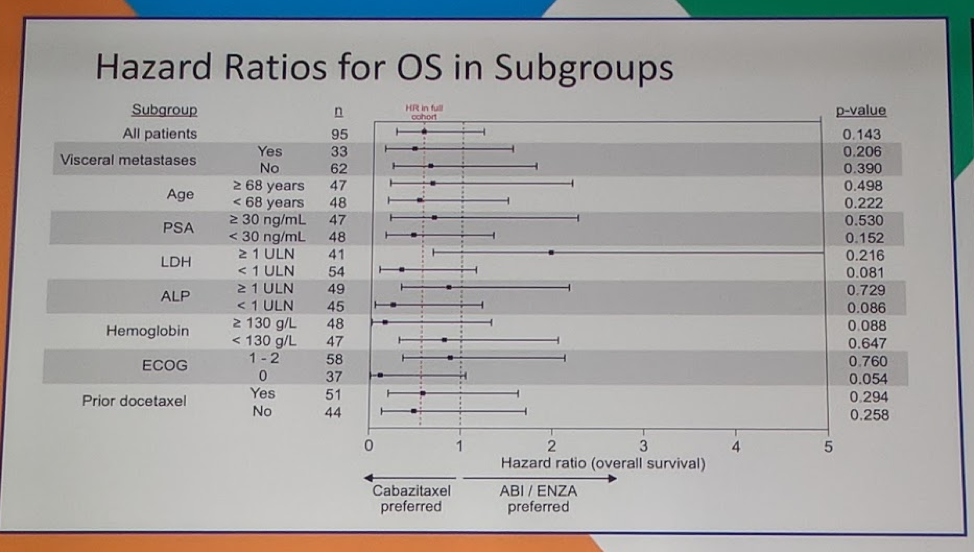
In terms of baseline characteristics, most factors were balanced including age, poor prognostic features, ECOG performance status, hemoglobin, alkaline phosphatase, LDH, and receipt of prior docetaxel. Only PSA was different between the two cohorts, with a median PSA of 39.4 in the abi/enza cohort, compared with 18.7 in the first line cabazitaxel cohort. In terms of clinical benefit for first-line therapy, a significantly higher number of patients benefited from receiving cabazitaxel over abi/enza (90% vs 70%, p=0.02). 57% of patients on cabazitaxel experienced a PSA50 (PSA decline of 50% or more) compared with 60% of patients on abi/enza (p=0.835). There was no difference in measurable disease response (17% vs 25%, p=0.724). In terms of second-line therapy once patients’ switched over, there was no difference in PSA50, measurable disease response, or stable disease >12 weeks (75% vs 85%, p=0.483) and ultimately there was no difference in overall survival (p=0.143) for the total cohort as well as several subgroups.
Correlative studies done in conjunction with this study demonstrated that ctDNA/cfDNA fraction was prognostic. Notably, no patients with undetectable ctDNA have died in the study. Patients with ctDNA percent of 30-100 had a HR for progression of 4.2 (95%CI 2.04-8.68, p<0.001). ctDNA change on therapy was also highly prognostic - ctDNA increase while on therapy had a HR of 6.24 (95%CI 2.09-16.63, p=0.001) for overall survival. 
AR amplification was studied as well. It has been suggested in prior studies that AR gene amplification may be one of the possible molecular mechanisms which lead to ADT failure6. In this study, AR amplification did not predict which patients would respond to cabazitaxel or to abi/enza.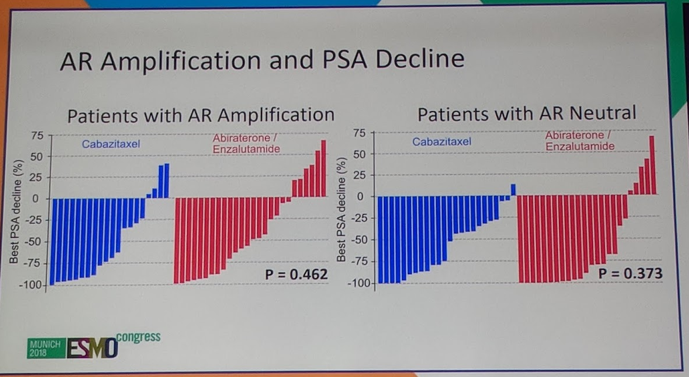
This study did not demonstrate an overall survival benefit with upfront cabazitaxel over abi/enz in a very poor prognosis subset of patients with mCRPC. However, about 50% of patients will not make it to chemotherapy as second line as shown in this study (only 28 patients of the 50 who received abi/enz got cabazitaxel), which may argue for earlier chemotherapy in the window of opportunity for benefit. The discussant does note that this study was underpowered to explore overall survival as the study stopped accrual early due to slow enrollment. One of the hypotheses of slow accrual may have been that clinicians and/or patients were uncomfortable with randomization. The clinical correlates from the study show that ctDNA change appears prognostic and may help risk stratify patients for more aggressive therapies in the future.
Presented by: Kim N. Chi, MD, Clinical Associate Professor, Medical Oncologist, BCCA, University of British Columbia, Vancouver, Canada
Invited Discussant: Stéphane Oudard, MD, Ph.D., Georges Pompidou European Hospital, Department of Medical Oncology, Paris, France
References:
1. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and Increased Survival in Metastatic Prostate Cancer. The New England journal of medicine 2011;364:1995-2005.
2. Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomized, double-blind, placebo-controlled phase 3 study. The Lancet Oncology 2015;16:152-60.
3. Scher HI, Fizazi K, Saad F, et al. Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. New England Journal of Medicine 2012;367:1187-97.
4. Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. The New England journal of medicine 2014;371:424-33.
5. Chi KN, Annala M, Sunderland K, et al. A randomized phase II cross-over study of abiraterone+ prednisone (ABI) vs enzalutamide (ENZ) for patients (pts) with metastatic, castration-resistant prostate cancer (mCRPC). American Society of Clinical Oncology; 2017.
6. Koivisto P, Kononen J, Palmberg C, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer research 1997;57:314-9.
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, Twitter: @TheRealJasonZhu at the 2018 European Society for Medical Oncology Congress (#ESMO18), October 19-23, 2018, Munich Germany


