Genomic stratification for precision medicine is an evolving science and treatment strategy. There are several important factors that need to be considered in planning the utilization of the strategy of precision medicine. These include the sample that is used, the assay, the interpretation that is done, and the match (hypothesis).
The hypothesis relevant for these three posters presented states that by implementing next-generation sequencing (NGS) assays, we can identify a population of mCRPC patients with DNA damage repair (DDR) gene defects, who would derive the biggest benefit from PARP inhibitors in a clinical trial.
The first poster discussed was the 794PD presented by Ali et al.: Genomic profiling of 3476 primary and metastatic prostate tumors. In this study, 1660 primary tumor samples and 1816 metastatic biopsies (29% nodal, 25% hepatic, and 22% bone) were taken from non-matched patients and evaluated using NGS. The objectives were to understand the genomic landscape in unpaired primary and metastatic prostate cancers. Additionally, the goal was to evaluate the genome-wide loss of heterozygosity (gLOH) and tumor mutational burden genomic signatures, and associations with genotype. LOH is a cross chromosomal event that results in loss of the entire gene and the surrounding chromosomal region. LOH is a common occurrence in cancer, where it indicates the absence of a functional tumor suppressor gene in the lost region. The remaining copy of the tumor suppressor gene can be inactivated by a point mutation, leaving no tumor suppressor gene to protect the body.
In the 794 study, various mutations were tested, as described in figure 1. There was a correlation between the mutations found in the primary tumor and metastatic lesions, with similar frequency in both anatomic location of the mutations in MYC, RB1, FGF3/4/19, PIK3CB, NCOR1, LYN, CCND1, PTEN, and TP53.
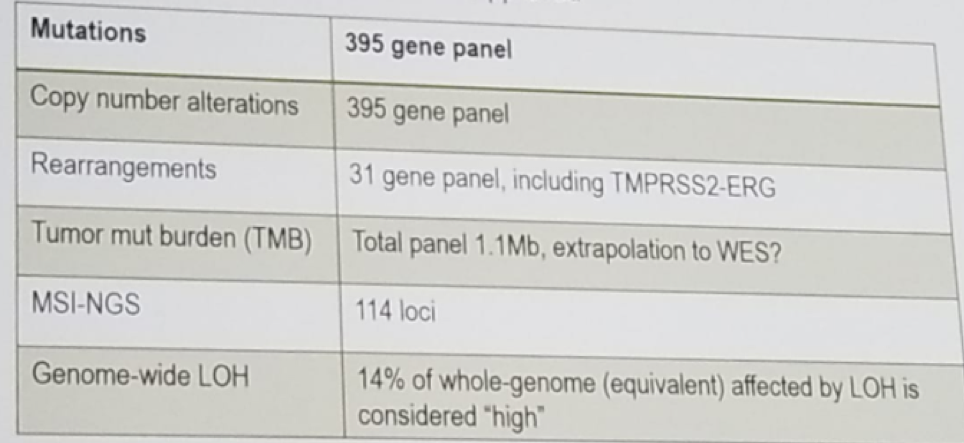
Figure 1- Mutations tested
Overall, 17% of the cases were gLOH-high, and 30-40% BRCA 1 and two alterations (both predicted germline and somatic) were frequently gLOH-high, possibly demonstrating biallelic BRCA loss. These findings raise some questions regarding whether gLOH might be changing in BRCA2 mutations and wild-type throughout the castration resistance pathway. Another question is whether this can be a stratification strategy for PARP inhibitors in CRPC patients? How many of the gLOH high have a DDR gene defect? And is it possible to define a clinically-relevant DDR+ signature?
Dr. Mateo continued and moved on to the next poster – 795PD: Chowdhury et al.: Screening of DDR mutations in CRPC cell-free DNA and tumor tissue for the TRITON 2 and three trials. This was a multicenter prospective NGS screening of CRPC patients using a primary tissue and metastatic tissue of plasma (cell-free DNA) to identify candidates for the Rucaparib (a PARP inhibitor) clinical trials TRITON2 and TRITON3 using the FoundationOne capture -based assay.
TRITON2 (NCT02952534) is a phase 2 study evaluating rucaparib 600 mg BID in patients with mCRPC. Patients with a deleterious germline or somatic BRCA1, BRCA2 or ATM mutation will be enrolled into 1 of 2 cohorts based on the presence or absence of measurable visceral and nodal disease. Patients must have progressed on androgen receptor signaling–directed therapy and one prior taxane-based chemotherapy for mCRPC. The primary endpoint is objective response rate.
TRITON3 (NCT02975934) is a randomized, phase 3 study evaluating rucaparib 600 mg BID vs. physician’s choice of abiraterone, enzalutamide, or docetaxel in patients with mCRPC and a deleterious germline or somatic BRCA1, BRCA2, or ATM mutation. Patients must have progressed on androgen deprivation therapy in the mCRPC setting. Patients will be randomly assigned in a 2:1 ratio to either rucaparib or physician’s choice, with the possibility for crossover from the comparator treatment to rucaparib upon radiographic progression confirmed by independent radiology review. The primary endpoint is radiographic progression-free survival.
In the 795PD study, there was a high failure rate in the NGS, with 26% of M1 biopsies, 44% of the primary tumor biopsies, and 3% of the plasma samples failing NGS. Looking at the results, 20 patients with BRCA 1 and 2 had homozygous loss mutations in the tissue, and these would not have been picked up by using only cell-free DNA testing from the plasma. The frequency of the various mutations in tissue and plasma are demonstrated in Figure 2. The only striking difference between the two environments is that the ATM mutation is much more prevalent in the plasma (14.6% and 8.2% vs. 6.6% and 5.7%, in the TRITON 2 and TRITON 3 studies, respectively).
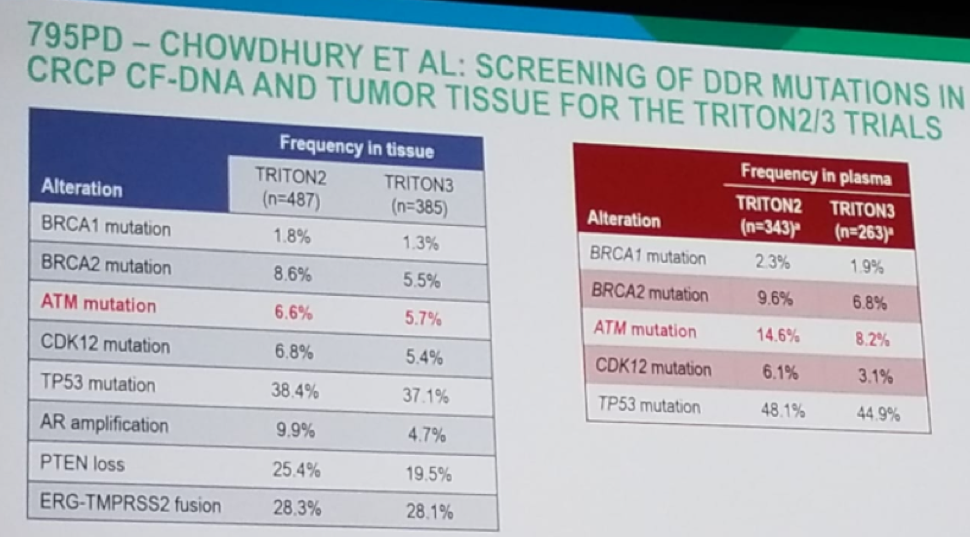
Figure 2 – Results of mutation in tissue and plasma:
This raises the questions whether the ATM mutation is a late event, heterogeneous in the tumor? And whether this will cause a clinically relevant difference when it comes to the impact the PARP inhibitor treatment will have.
The last poster discussed was 793PD – Abida et al.: Preliminary results of the TRITONE2- Rucaparib in CRPC patients with DDR mutations. It is important to note that the results should be read carefully, as this is not a pre-planned interim analysis of the TRITON2 study, and only provides data on 85 patients. As mentioned earlier, the TRITON 2 study (NCT02952534) is a phase 2 study evaluating rucaparib 600 mg BID in patients with mCRPC. Patients with a deleterious germline or somatic BRCA1, BRCA2 or ATM mutation will be enrolled into 1 of 2 cohorts based on the presence or absence of measurable visceral and nodal disease. The primary endpoint is objective response rate. The results demonstrated an overall response rate of 44% in BRCA 1 and two mutations, with a confirmed PSA response rate of 51.1%. These very preliminary results are quite impressive when comparing them to those of the abiraterone trials (overall response rate of 14% and PSA response rate of 38%) and the enzalutamide trials (overall response rate of 29% and PSA response rate of 54%). There have also been confirmed responses in patients with FANCA, CDK12, and BRIP1 mutations. The adverse events demonstrated that anemia was the most common grade 3-4 adverse event (15%), with a very common prevalence of gastrointestinal adverse effects (grade 1-2 only).
These preliminary TRITON 2 results are quite promising, and Dr. Mateo stated that he is quite eager to see the final trial results, and especially looking forward to seeing the confirmed response rates with the full trial population and analyze the durability of the benefit.
Dr. Mateo concluded his talk with some take-home messages. NGS is making its way into prostate cancer in a steady and gradual manner, with significant advances over the last five years. Up to this point, several studies have already shown that the prevalence of BRCA1 and BRCA 2 defects in CRPC patients is approximately 10-12%, with a bigger list of DDR mutations in almost 25% of CRPC patients. Plasma cell-free DNA is a promising tool for genomic stratification, but it still needs to be optimized before it can be introduced into routine care. A key element of success in its utilization is understanding the evolution and heterogeneity of the mutations. Lastly, the PARP inhibitor, Rucaparib, had demonstrated promising anti-tumor activity in BRCA 1 and two deficient prostate cancer. Longer follow-up and larger patient numbers are needed to better asses the full magnitude of the effect of this medication.
Presented by: Joaquin Mateo, MD, Ph.D., Vall d’Hebron Institute of Oncology, Barcelona, Spain
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, Twitter: @GoldbergHanan at the 2018 European Society for Medical Oncology Congress (#ESMO18), October 19-23, 2018, Munich Germany


