- 796PD - Detection of circulating tumor DNA in de novo metastatic castrate-sensitive prostate cancer – Werner Strauss et al.
- 797PD - LATITUDE study: PSA response characteristics and correlation with overall survival (OS) and radiological progression-free survival in patients with metastatic hormone-sensitive prostate cancer receiving ADT+abiraterone acetate and prednisone or placebo – Nobiaki Matsubara et al.
- It is not evident that these treatments are adequate and equal for all patients.
- The stratification of high/low volume disease and its implication on the type of treatment that should be given is still an open debate.
- Which one to use first? Abiraterone or docetaxel?
- Is there value in the molecular profiling of a tumor?
In this study, a total of 90% of patients had Gleason 8-10 disease, and 75% had bone metastases. Circulating tumor DNA (ctDNA) was detected in most cases with visceral metastases being shown to be correlated with higher ctDNA counts. In contrast, androgen deprivation therapy (ADT) significantly reduced the fractions of ctDNA. The concordance for mutation detection in matched samples was 80%. Combined ctDNA and tissue analysis identified alterations in 94% of the cohort, whereas ctDNA or prostate biopsy alone was insufficient in 19 cases (36%).
Summarizing the results from this study, ctDNA is detectable in mHSPC patients, but ADT rapidly reduces ctDNA availability. That is why it is important to get patient samples before starting treatment with ADT. A combination of tissue and liquid biopsy may be required to get all the information, and the value of this approach for prognosis/treatment is currently not clear.
Dr. O’Sullivan continued to discuss the next study – 797PD - LATITUDE study: PSA response characteristics and correlation with overall survival (OS) and radiological progression-free survival in patients with metastatic hormone-sensitive prostate cancer receiving ADT+abiraterone acetate and prednisone or placebo – Nobiaki Matsubara et al. The LATITUDE study (Figure 1) was a prospective randomized phase 3 trial randomizing newly diagnosed mHSPC men to either abiraterone and ADT or placebo and ADT. The study showed a clear benefit to the abiraterone arm in PSA progression and overall survival. Patients with a greater PSA response also showed better survival rates (Figure 2).
Figure 1- LATITUDE study design:

Figure 2 – Better responders live longer according to the LATITUDE trial:
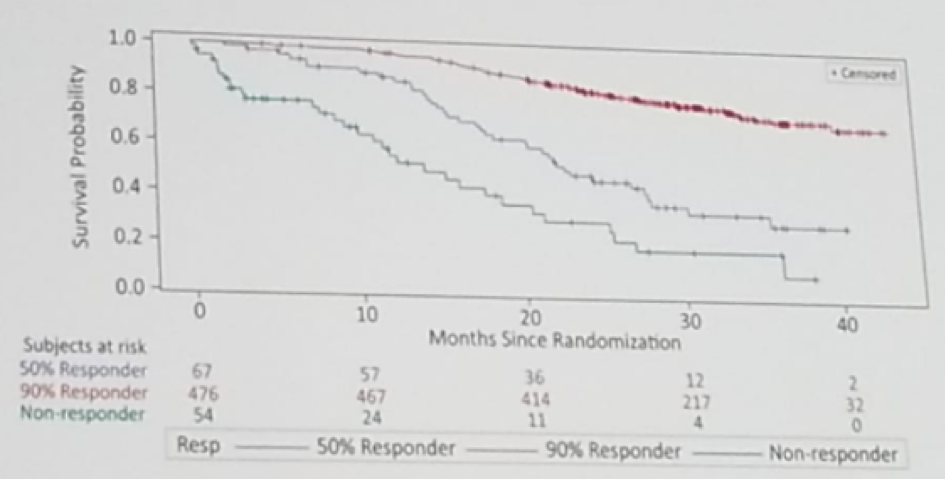
The LATITUDE study also demonstrated that the time to PSA nadir correlates with radiographic progression-free survival. Patients that took less than six months to reach their PSA nadir, had a median time to death of 29.57 months, while patients that took more than 12 months to reach their PSA nadir did not reach the median time to death. This means that it takes longer to reach lower PSAs, and lower PSAs correlate with better outcomes.
Summarizing the results, PSA response correlates with radiographic progression-free survival and overall survival. This raises the question, whether this could be a surrogate marker for overall survival and radiographic free survival in trials. However, the clinical usefulness of this marker is unclear, as a time to nadir and the extent of nadir can only be determined retrospectively. We need to see how the biology could be correlated with PSA/radiological response.
Presented by: Joe O'Sullivan, MD, Queen's University Belfast, Great Britain
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, Twitter: @GoldbergHanan the 2018 European Society for Medical Oncology Congress (#ESMO18), October 19-23, 2018, Munich Germany


