While most experts agree that abiraterone is reasonable to use for patients with high-risk metastatic CSPC, what to do for patients with low-risk metastatic disease is debatable. This study seeks to answer that important question. Before we go into the trial, it is important to recognize that the definition of high risk or high volume disease is different depending on the trial. In CHAARTED3, high volume disease was defined as visceral metastases or ≥4 bone metastases with one or more of the bone metastases outside the vertebral column or pelvis. The criteria was different in LATITUDE, which defined high-risk patients as 2 or more of the following features: ≥3 bone metastases, visceral metastases, Gleason ≥8.

This study evaluates the efficacy of abiraterone in low-risk mCSPC by retrospectively stratifying M1 STAMPEDE patients into high and low risk disease. The primary stratification used was the LATITUDE criteria and CHAARTED criteria were used as an exploratory endpoint.
Almost 990 patients were included in this study out of the 1917 original STAMPEDE patients. The steep drop-off was mainly due to 914 patients who were non-metastatic. This left 452 in the standard of care (SOC) arm and 449 in the SOC + abiraterone arm.
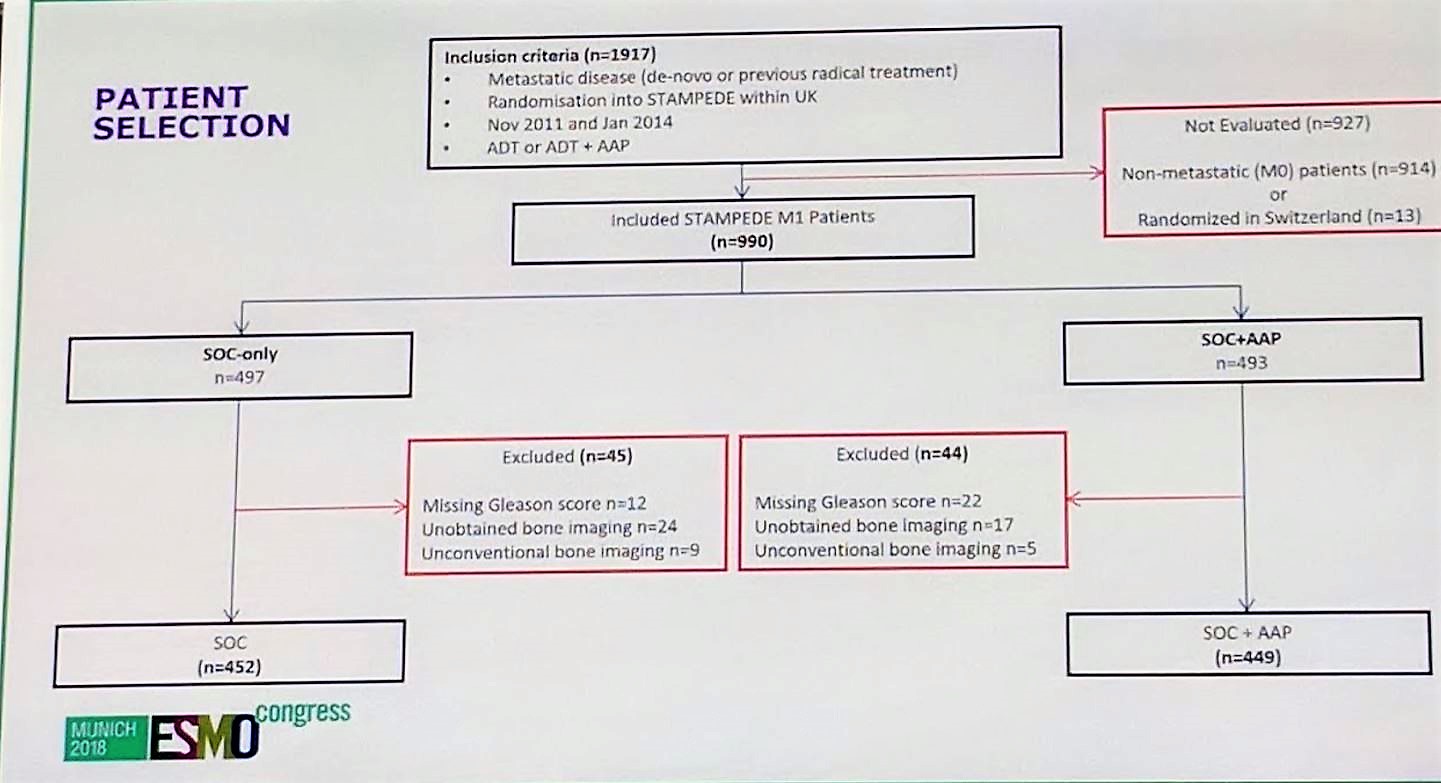
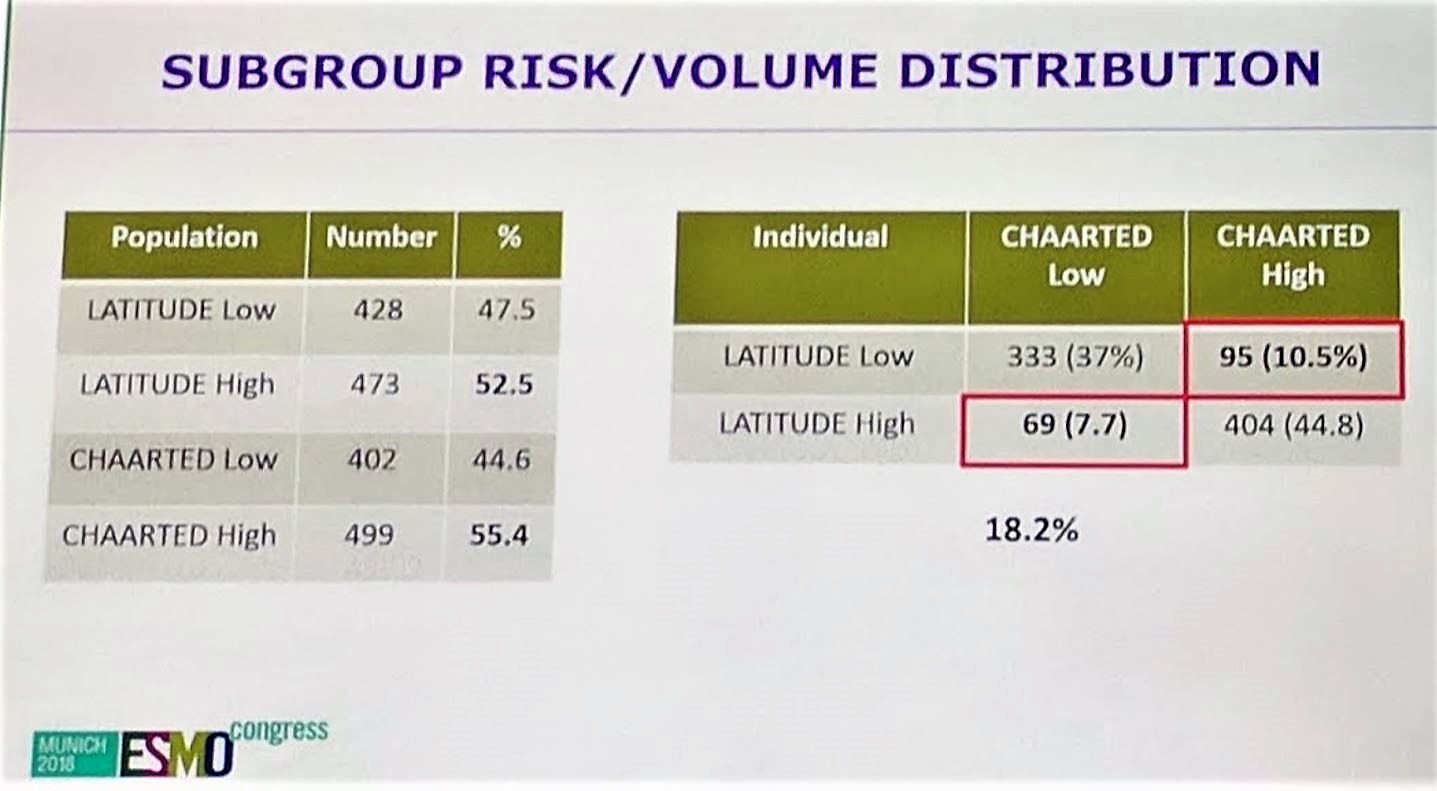
Next, patients in each arm were stratified by the LATITUDE and CHAARTED criteria. Of note, 18.2% of all patients would have discordant stratification, depending on which criteria was used. It is important to clearly state the criteria in future trials as this could potentially impact outcomes.
Baseline characteristics are shown below. There were no significant differences between age, WHO PS, tumor stage, nodal stage, or sites of disease between the high risk and low risk population. Almost all patients were de novo metastatic and investigators have significant follow up, with a median of 41.5 months.

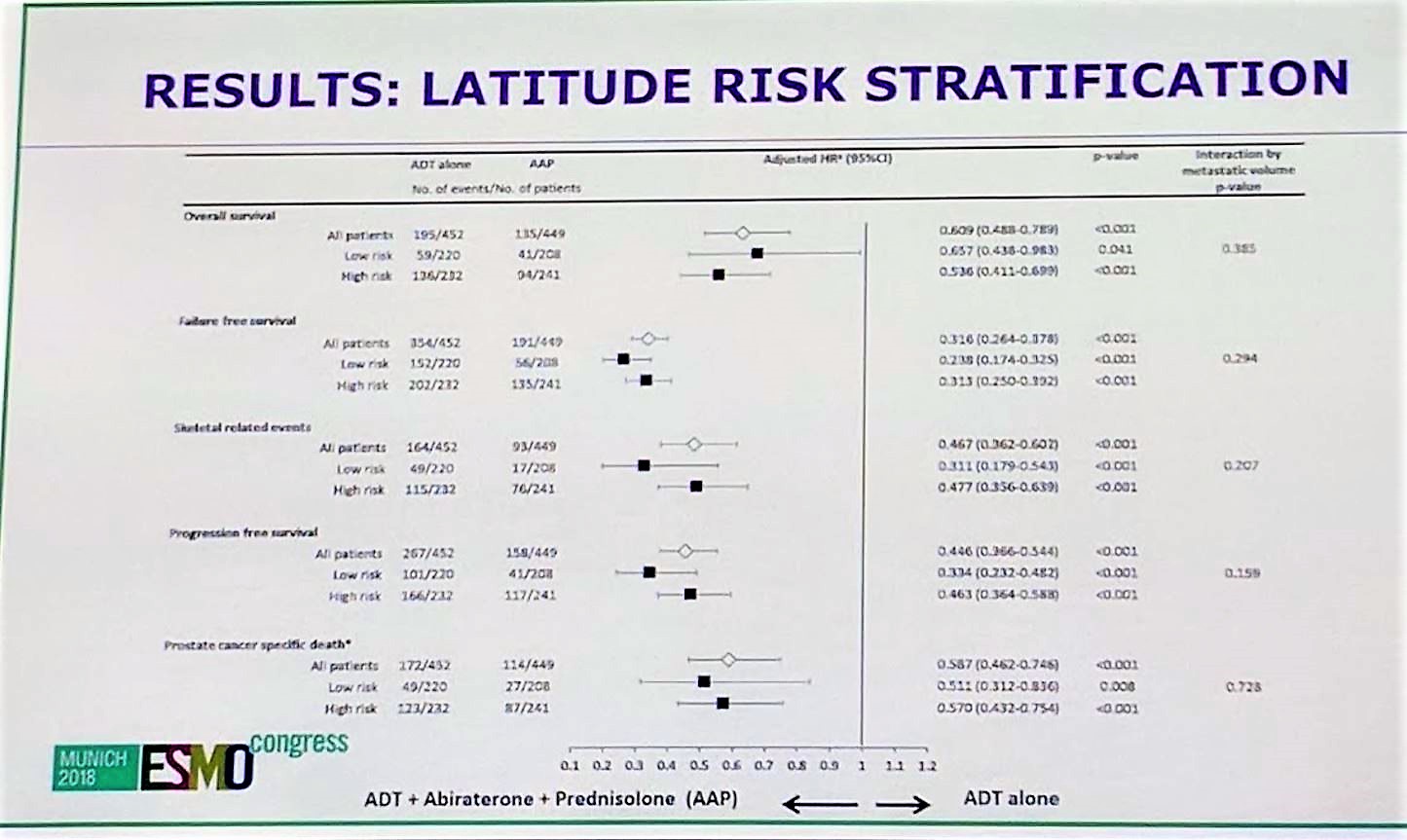
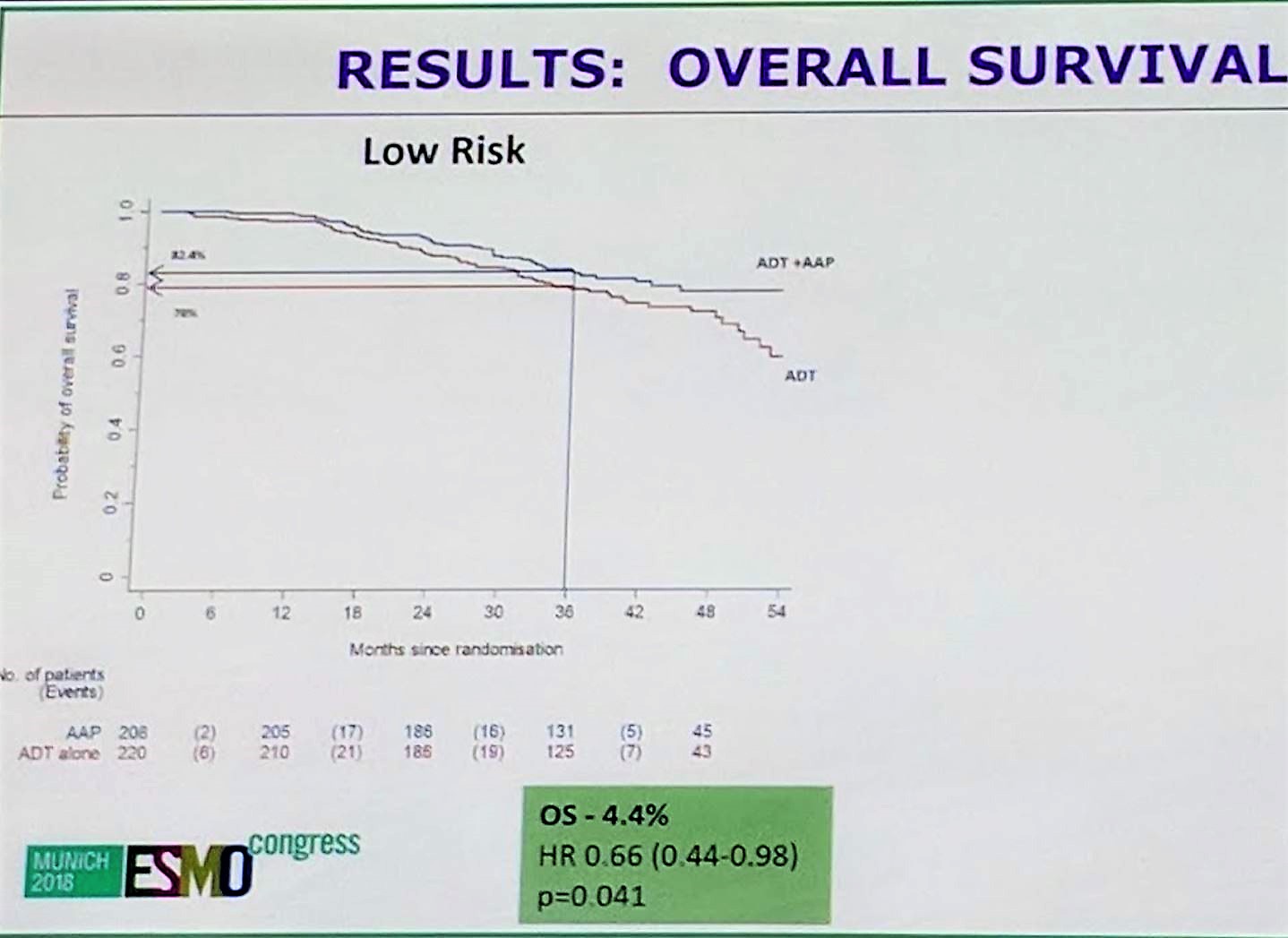
In terms of overall survival, abiraterone + SOC demonstrated benefit over SOC in both high and low risk subgroups, with a HR of 0.657 for low risk and HR of 0.536 for high risk. In addition to overall survival, both groups benefited from failure free survival, skeletal related events, progression free survival, and prostate cancer specific deaths. P values for interaction were all non-significant, suggesting that the benefit of abiraterone + SOC is consistent throughout all subgroups between high and low risk disease.
The same analysis was repeated but with CHAARTED volume criteria, and again, abiraterone demonstrated benefit over placebo for patients with both high volume and low volume disease.

This retrospective analysis of almost 1000 patients who had previously been randomized to SOC or SOC + Abiraterone demonstrates that both high risk/high volume patients and low risk/low volume patients with mCSPC benefit from abiraterone, in terms of overall survival, progression free survival, metastasis free survival, and skeletal related event free survival. These results are practice changing and suggest that all patients with mCSPC should be treated with ADT plus Abiraterone up front.

The data presented from LATITUDE does not address what we should do for low volume metastatic patients who go on to receive radiation therapy. PEACE-1 will help us answer that important question. PEACE-1 is a randomized clinical trial which investigates four different therapies for the initial treatment of newly diagnosed metastatic CSPC: ADT, ADT+Abiraterone 1000 mg daily + prednisone 5 mg twice a day, ADT+local radiotherapy, and ADT+local radiotherapy+Abiraterone+predisone. Data from PEACE-1 will complement the evidence provided today to help us better take care of our patients with mCSPC.
Presented By: Alex P. Hoyle, MBChB, MRCS, The Christie and Salford Royal Hospitals, Manchester, United Kingdom
Written By: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, Twitter: @TheRealJasonZhu at the 2018 European Society for Medical Oncology Congress (#ESMO18), October 19-23, 2018, Munich Germany
References:
1. Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. New England Journal of Medicine 2017;377:352-60.
2. James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. New England Journal of Medicine 2017;377:338-51.
3. Sweeney CJ, Chen Y-H, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. New England Journal of Medicine 2015;373:737-46.


